Volume 19, Number 10—October 2013
Letter
Multidrug-Resistant Escherichia coli Bacteremia
To the Editor: Extraintestinal pathogenic Escherichai coli (ExPEC) bacteria have the ability to cause diverse and serious diseases, such as urinary tract infections (UTIs) and bacteremia (1–3); incidence of bacteremia is increasing globally (4). The emergence of multidrug resistance in E. coli is also becoming a global concern, with particular emphasis on E. coli sequence type (ST) 131, which is being increasingly reported in UTIs. Drug resistance is mediated by extended-spectrum β-lactamases (ESBLs), mainly of the CTX-M family, particularly CTX-M-15 and 14, and less frequently of the SHV and OXA families (5,6). Few studies are available regarding the characterization of E. coli strains causing bacteremia.
We characterized 140 E. coli isolates from bacteremia patients treated at Nottingham University Hospital (Nottingham, UK) over a 5-month period, with the aim of developing an epidemiologic profile of the population of ExPEC that causes bacteremia. For context, we compared the isolates with 125 E. coli isolates from urine samples collected during the same period. Cases were selected to include isolates from a diverse patient group: patient ages ranged from 1 month to 90 years; patient sex was evenly divided between male and female; infections were community- and hospital-associated; and suspected sources of infection varied. Antimicrobial drug susceptibility tests, PCR detection of ESBL genes, multilocus sequence typing using the Achtman scheme (http://mlst.ucc.ie/mlst/dbs/Ecoli), and virulence-associated gene (VAG) carriage screening by PCR were performed on isolates as described (7).
Significantly more bacteremia E. coli isolates than urine E. coli isolates were resistant to ciprofloxacin (25.7% vs. 8.8%; p<0.001) and cefradine (20.0% vs. 11.2%; p<0.05). These results were reflected in the number of isolates in the 2 populations displaying a multidrug- resistance phenotype (resistance to antimicrobial drugs belonging to >2 classes); a significantly higher number of multidrug-resistant bacteremia E. coli isolates than multidrug-resistant urine isolates were found (50.7% vs. 32%; p = 0.01). PCR screening for ESBL carriage showed significantly higher ESBL carriage in bacteremia E. coli isolates than urine isolates for blaSHV (15.7% vs. 5.6%; p = 0.008), blaCTX-M (29.3% vs. 17.6%; p = 0.025), and blaOXA (14.3% vs. 6.4%; p = 0.037). Total ESBL carriage for bacteremia isolates was also significantly higher than for urine isolates (59.3% vs. 29.6%; p<0.001).
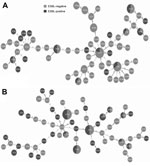
Multilocus sequence types were determined for all E. coli isolates. A total of 63 STs were found among the urine isolates (Figure, panel A); the highest prevalence was ST73 (n = 16, 12.8%), followed by ST131 (n = 9, 7.2%), ST69 (n = 9, 7.2%), ST95 (n = 6, 4.8%), ST404 (n = 6, 4.8%), ST127 (n = 4, 3.2%), ST141 (n = 4, 3.2%), and ST10 (n = 3, 2.4%). Prevalence patterns of STs among bacteremia E. coli isolates were noticeably different (Figure, panel B). Three main STs were obtained. ST131 dominated (n = 30, 21.43%) and was significantly higher in prevalence than for the urine isolates (p<0.001). ST73 (n = 24, 17.14%) and ST95 (n = 13, 9.29%) were the other 2 primary STs found. The 8 most prevalent STs in the bacteremia isolates represented 59.29% of the total population, whereas the 8 most prevalent STs in the urine isolates represented 45.6% of the total population. This finding is suggestive of selection of a smaller number of dominant STs in bacteremia.
ESBL carriage was mapped onto minimum-spanning trees for the 2 isolate groups. ESBL carriage among urine isolates was focused on a small number of STs; 19 (30.16%) of the 63 STs contained ESBL-positive isolates (Figure, panel A). The predominant ST73 group contained 18.75% ESBL-positive isolates; the other predominant STs exhibited ESBL-positive isolates at the following levels: ST131 (44.44%), ST69 (33.33%), ST95 (50%), and ST10 (0%). In contrast, 30 (51.72%) of the 58 STs among bacteremia isolates contained ESBL-positive isolates, significantly higher than for the urine isolates (p = 0.016). At the ST level, predominant STs had higher ESBL carriage in the bacteremia isolates than in the urine isolates: ST131 (50%), ST73 (50%), ST12 (75%), ST10 (100%), ST14 (50%), ST2278 (33.33%). ST95 (46.15%) and ST69 (20%) showed comparable levels. These results suggest that ESBL drug resistance is selecting for dominant ExPEC bacteremia strains.
To investigate whether the differences in ST observations between bacteremia and urine isolates could be attributable to differences in virulence genes, VAGs of all isolates were screened by multiplex PCR. VAGs were found equally distributed across the 2 populations, with no statistically significant difference (p = 0.675). Comparison of serum resistance levels between urine and blood isolates also showed no phenotypic differences.
In conclusion, we found high levels of ESBL carriage and multidrug resistance in ExPEC isolates that cause bacteremia. A comparison with urine isolates provided evidence that ESBL-mediated drug resistance appears to be the selective pressure in the emergence of dominant STs in bacteremia. Future research should focus on identifying if prolonged antimicrobial drug treatment in bacteremia patients is leading to this selection.
Acknowledgments
We thank staff in the clinical microbiology laboratory at Nottingham University Hospitals for their assistance in isolate collection and Nicholas Gleadall and Kuan Min Chen for technical assistance in performing multilocus sequence typing PCRs.
This work was supported by a Kuwait Government and Kuwait Civil Service Commission scholarship awarded to F.A. and an East Midlands Development Agency iNET grant awarded to A.M. and M.D.
References
- Wiles T, Kulesus R, Mulvey M. Origins and virulence mechanisms of uropathogenic Escherichia coli. Exp Mol Pathol. 2008;85:11–9. DOIPubMedGoogle Scholar
- Ron EZ. Distribution and evolution of virulence factors in septicemic Escherichia coli. Int J Med Microbiol. 2010;300:367–70. DOIPubMedGoogle Scholar
- Russo TA, Johnson J. Medical and economic impact of extraintestinal infections due to Escherichia coli: focus on an increasingly important endemic problem. Microbes Infect. 2003;5:449–56 . DOIPubMedGoogle Scholar
- de Kraker ME, Jarlier V, Monen JC, Heuer OE, van de Sande N, Grundmann H. The changing epidemiology of bacteremias in Europe: trends from the European Antimicrobial Resistance Surveillance System. Clin Microbiol Infect. 2012; [Epub ahead of print].
- Nicolas-Chanoine MH, Blanco J, Leflon-Guibout V, Demarty R, Alonso MP, Canica MM, Intercontinental emergence of Escherichia coli clone O25:H4–ST131 producing CTX-M-15. J Antimicrob Chemother. 2008;61:273–81 . DOIPubMedGoogle Scholar
- Lau SH, Kaufmann ME, Livermore DM, Woodford N, Willshaw GA, Cheasty T, UK epidemic Escherichia coli strains A–E, with CTX-M-15 β-lactamase, all belong to the international O25:H4–ST131 clone. J Antimicrob Chemother. 2008;62:1241–4. DOIPubMedGoogle Scholar
- Croxall G, Hale J, Weston V, Manning G, Cheetham P, Achtman M, Molecular epidemiology of extraintestinal pathogenic Escherichia coli isolates from a regional cohort of elderly patients highlights the prevalence of ST131 strains with increased antimicrobial resistance in both community and hospital care settings. J Antimicrob Chemother. 2011;66:2501–8 . DOIPubMedGoogle Scholar
Figure
Cite This ArticleRelated Links
Table of Contents – Volume 19, Number 10—October 2013
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Alan McNally, Pathogen Research Group, School of Science and Technology, Nottingham Trent University, Clifton Lane, Nottingham NG11 8NS, UK
Top