Volume 19, Number 11—November 2013
Dispatch
Severe Fever with Thrombocytopenia Syndrome, South Korea, 2012
Cite This Article
Citation for Media
Abstract
We report a retrospectively identified fatal case of severe fever with thrombocytopenia syndrome (SFTS) in South Korea from 2012. SFTS virus was isolated from the stored blood of the patient. Phylogenetic analysis revealed this isolate was closely related to SFTS virus strains from China and Japan.
Severe fever with thrombocytopenia syndrome (SFTS) causes signs and symptoms including high fever, vomiting, diarrhea, thrombocytopenia, leukopenia, and multiple organ failure and has a 6%–30% case-fatality rate (1–4). Caused by a novel bunyavirus, SFTS virus (SFTSV), SFTS was initially reported in China in 2011 (1). SFTSV has been detected in Haemaphysalis longicornis ticks, which have been implicated as a vector of the virus (1). H. longicornis ticks widely inhabit the Korean Peninsula (5,6), and the Korea Centers for Disease Control and Prevention reported that SFTSV was detected in samples from H. longicornis ticks collected during 2011–2012 in South Korea (7). Seroconversion and viremia of SFTSV have been demonstrated in domesticated animals such as goats, sheep, cattle, pigs, and dogs; these animals have been implicated as intermediate hosts in SFTSV-endemic areas (8,9). SFTSV was also detected in Japan in February 2013 (10). We report a retrospectively identified case of SFTS in South Korea from 2012 and the characterization of the SFTSV isolated from the patient.
On August 3, 2012, fever developed in a previously healthy 63-year-old woman who lived in Chuncheon-si, Gangwon Province, South Korea; the same day, she noticed a lump on the left side of her neck. She visited a local clinic, and ciprofloxacin and ceftriaxone were started on the first day of illness. The patient reported that, 2 weeks before her fever started, she noticed an insect bite on her neck while she was working on a crop farm in Hwacheon-gun, Gangwon Province (in the northernmost part of South Korea). She did not recall having contact with any domestic animals on the farm and had no history of travel outside South Korea in the month before illness onset.
On the third day of her illness, she began having watery diarrhea, 6 times per day. On the fourth day of the illness, thrombocytopenia and leukopenia were recorded at the local clinic (Table). Because of worsening thrombocytopenia, she was transferred to another hospital. Ciprofloxacin was changed to doxycycline, and ceftriaxone was continued. A computed tomography scan of the neck showed an enlarged (1.6 cm), necrotic lymph node. Multiple lymph nodes on the left cervical and left axillary areas were also swollen. On the sixth day, the patient was transferred to Seoul National University Hospital.
At admission to the hospital, the patient was febrile but alert. Her temperature was 38.7°C, blood pressure 126/70 mm Hg, heart rate 86 beats per minute, and oxygen saturation 92% on room air. Her face was puffy, with a sunburned appearance, and both conjunctivae were congested. The insect bite site on her posterior neck was swollen and erythematous, and the draining cervical lymph node was enlarged. Petechiae were observed on her shoulders and lower extremities.
Laboratory test results showed pancytopenia and elevated serum aminotransferase levels; prothrombin and activated partial thromboplastin times were normal, but fibrinogen level was decreased (Table). A urine dipstick test showed albuminuria (+++), and microscopic examination of the urine revealed >100 erythrocytes per high-power field. Test results for antibodies against Orientia tsutsugamushi, Hantaan virus, and leptospira were negative. A chest radiograph showed bilateral increased vascular markings, and the plasma level of B-type natriuretic peptide increased to 134 pg/mL (reference range <100 pg/mL); these findings suggested cardiac dysfunction.
On the eighth day of her illness, the patient spoke incoherently and was unable to communicate. Cerebrospinal fluid analysis showed no erythrocytes or leukocytes and a normal chemistry profile. A computed tomography scan of the brain showed no evidence of hemorrhage or infarction and no other abnormalities. She was transferred to the intensive care unit. On the ninth day, she was intubated and placed on continuous renal replacement therapy. On the tenth day of illness (August 12, 2012), the patient died of multiple organ failure. Ceftriaxone and doxycycline were continued until the patient’s death. Antiviral drugs, corticosteroids, immunosuppressive agents, or intravenous immunoglobulin were not given.
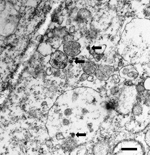
Because viral infection was suspected but no virus could be identified, an anticoagulated blood sample was obtained from the patient on the eighth day of illness and stored at −70°C. When testing for SFTSV became available 7 months later, we inoculated monolayers of Vero cells with the patient’s blood sample and cultured the cells at 37°C in a 5% carbon dioxide atmosphere. A culture supernatant obtained 13 days after the inoculation was used for genetic analysis. The culture supernatant was also used to inoculate DH82 cells when the cell line became available; 5 days after the inoculation, we observed a cytopathic effect of SFTSV in DH82 cells. The SFTSV-infected Vero cell monolayer was fixed according to described methods (11) and cut on ultramicrotome (RMC MT-XL) at 65 nm. Ultrathin sections were stained with saturated 4% uranyl acetate and 4% lead citrate before examination with a transmission electron microscope (HITACHI-7100; Hitachi High-Technologies, Ibaraki, Japan) at 75 kV (Figure 1).
RNA was extracted from the stored blood and from virus-infected Vero cells by using a QIAamp Viral RNA Mini Kit (QIAGEN, Hilden, Germany). Reverse transcription PCR (RT-PCR) was performed to amplify the partial large (L) segment of the viral RNA from the stored blood to confirm SFTSV, as described (12). RT-PCR results were positive, and direct sequencing was done. A BLAST search (http://blast.ncbi.nlm.nih.gov/Blast.cgi) showed no sequences from organisms other than SFTSV.
Using the culture supernatant, full lengths of all 3 genome segments (L, medium [M], and small [S]) were sequenced by RT-PCR and direct sequencing was performed by using primers designed from previously published SFTSV sequences. After polyadenylation of 3′ ends of the genomic and complementary RNAs, the sequences of the segment ends were obtained by rapid amplification of cDNA ends. The complete sequences of the L, M, and S segments were deposited in GenBank (accession nos. KF358691–KF358693). Sequences that had homology to our isolate were identified by BLAST search. The L, M, and S segments of the isolate showed 95.8%–99.8%, 94.1%–99.9%, and 94.8%–99.7% identity, respectively, to previously reported SFTSV sequences. We also constructed a phylogenetic tree by the neighbor-joining method using RNA-dependent RNA polymerase gene nucleic acid sequences to compare the isolate we obtained to representative SFTSV strains from China and Japan; the isolate and the other strains were closely related (95.9%–99.9% sequence relatedness) but not identical (Figure 2).
We confirmed a case of SFTS in South Korea in 2012 by isolation of SFTSV from a stored blood sample collected shortly before the patient’s death. The patient had a history of an insect bite while working on a crop farm in Hwacheon-gun, Gangwon Province, the northernmost part of South Korea. Phylogenetic analysis of the RNA-dependent RNA polymerase gene showed that our virus isolate was closely related to SFTSV strains reported from China and Japan.
As of July 5, 2013, the Korea Centers for Disease Control and Prevention had confirmed 13 cases of SFTS by RT-PCR; of these patients, 8 were dead and 5 alive (13). Except for our patient, who died in 2012, all cases occurred during 2013.
Dr Kye-Hyung Kim is an infectious disease physician and a senior researcher at Seoul National University College of Medicine, Seoul, South Korea. Her main research interests are medical virology and infectious disease epidemiology.
References
- Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011;364:1523–32. DOIPubMedGoogle Scholar
- Ding F, Zhang W, Wang L, Hu W, Soares Magalhaes RJ, Sun H, Epidemiologic features of severe fever with thrombocytopenia syndrome in China, 2011–2012. Clin Infect Dis. 2013;56:1682–3. DOIPubMedGoogle Scholar
- Zhang YZ, Zhou DJ, Xiong Y, Chen XP, He YW, Sun Q, Hemorrhagic fever caused by a novel tick-borne Bunyavirus in Huaiyangshan, China. Zhonghua Liu Xing Bing Xue Za Zhi. 2011;32:209–20 .PubMedGoogle Scholar
- Gai ZT, Zhang Y, Liang MF, Jin C, Zhang S, Zhu CB, Clinical progress and risk factors for death in severe fever with thrombocytopenia syndrome patients. J Infect Dis. 2012;206:1095–102. DOIPubMedGoogle Scholar
- Chae JS, Do-H Y, Shringi S, Klein TA, Kim HC, Chong ST, Microbial pathogens in ticks, rodents and a shrew in northern Gyeonggi-do near the DMZ, Korea. J Vet Sci. 2008;9:285–93. DOIPubMedGoogle Scholar
- Kim CM, Yi YH, Yu DH, Lee MJ, Cho MR, Desai AR, Tick-borne rickettsial pathogens in ticks and small mammals in Korea. Appl Environ Microbiol. 2006;72:5766–76. DOIPubMedGoogle Scholar
- The Korea Centers for Disease Control and Prevention. Prevention of severe fever with thrombocytopenia syndrome [in Korean]. 2013 May 2 [cited 2013 Jun 24]. http://www.cdc.go.kr/CDC/notice/CdcKrIntro0201.jsp?menuIds=HOME001-MNU0005-MNU0011&fid=21&q_type=&q_value=&cid=20790&pageNum=1
- Zhao L, Zhai S, Wen H, Cui F, Chi Y, Wang L, Severe fever with thrombocytopenia syndrome virus, Shandong Province, China. Emerg Infect Dis. 2012;18:963–5. DOIPubMedGoogle Scholar
- Niu G, Li J, Liang M, Jiang X, Jiang M, Yin H, Severe fever with thrombocytopenia syndrome virus among domesticated animals, China. Emerg Infect Dis. 2013;19:756–63 .PubMedGoogle Scholar
- ProMEDmail. Severe fever with thrombocytopenia syndrome—Japan (05): update. ProMed. 2013 Apr 10 [cited 2013 May 26]. http://www.promedmail.org, archive no. 20130410.1636456.
- Popov VL, Chen SM, Feng HM, Walker DH. Ultrastructural variation of cultured Ehrlichia chaffeensis. J Med Microbiol. 1995;43:411–21. DOIPubMedGoogle Scholar
- Liu Y, Li Q, Hu W, Wu J, Wang Y, Mei L, Person-to-person transmission of severe fever with thrombocytopenia syndrome virus. Vector Borne Zoonotic Dis. 2012;12:156–60. DOIPubMedGoogle Scholar
- ProMEDmail. Severe fever with thrombocytopenia syndrome—South Korea (05): additional fatalities. ProMed. 2013 Jul 6 [cited 2013 Jul 23]. http://www.promedmail.org, archive no. 20130706.1810682.
Figures
Table
Cite This Article1These authors contributed equally to this article.
Table of Contents – Volume 19, Number 11—November 2013
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Myoung-don Oh, Department of Internal Medicine, Seoul National University College of Medicine, 103 Daehak-ro, Jongno-gu, Seoul 110-744, South Korea
Top