Volume 19, Number 2—February 2013
Dispatch
Human Infection with Rickettsia sibirica mongolitimonae, Spain, 2007–2011
Abstract
Human infection with Rickettsia sibirica mongolitimonae was initially reported in 1996, and reports of a total of 18 cases have been published. We describe 6 additional cases that occurred in the Mediterranean coast region of Spain during 2007–2011. Clinicians should consider this infection in patients who have traveled to this area.
The genus Rickettsia contains ≈25 validated species of bacteria; another 25 isolates that have not been fully characterized or have not received a species designation have also been described. Signs and symptoms of human rickettsiosis caused by spotted fever group Rickettsia spp. include an inoculation eschar (a necrotic area at the site of the tick bite that might not be always present), fever, local adenopathies, and rash, although some variability can be found, depending on the infecting Rickettsia species.
R. sibirica mongolitimonae (also spelled mongolotimonae) was isolated from a Hyalomma asiaticum tick collected in the Alashian region of Inner Mongolia in 1991 (1). Designated R. mongolitimonae, the organism was identified as a member of the R. sibirica species complex (2), but further phylogenetic analyses grouped it in a cluster separate from other strains of R. sibirica.
The first human case of infection with R. sibirica mongolitimonae was reported in France in 1996 (3); since then, 18 additional cases have been described in the literature (4–14). Clinical signs and symptoms of infection are fever; a discrete, maculopapular rash; and enlarged regional lymph nodes, with or without lymphangitis. Although R. sibirica mongolitimonae infection causes a mild, not fatal, disease, complications such as acute renal failure and retinal vasculitis have been noted (7,10). We report 6 cases of human R. sibirica mongolitimonae infection from the same geographic region of Spain.
During July 2007–July 2011, six patients from the Mediterranean coast city of Elche, Spain, who had high fever and inoculation eschars received a diagnosis of infection with R. sibirica mongolitimonae (Table). For laboratory confirmation, DNA was extracted from eschars, lymph nodes (fine-needle aspiration), and blood samples by using the QIAamp Tissue Kit (QIAGEN, Hilden, Germany), according to the manufacturer’s instructions. For molecular detection, 200–400 ng of DNA from each sample was subjected to PCR targeting the 23S-5S rRNA intergenic spacer, followed by hybridization with specific probes by reverse line blotting, as described (15). When using the probe for R. sibirica mongolitimonae, a positive hybridization signal was obtained from eschar samples from all 6 patients; this result was confirmed by sequencing (100% similarity to a reference R. sibirica mongolitimonae strain [GenBank accession no. HQ710799] in all cases in the 357 bp sequenced). To further confirm this result, nested PCR targeting the gene for outer membrane protein A was performed as described (15); these sequences (514 bp) also showed 100% similarity to a reference R. sibirica mongolitimonae strain (GenBank accession no. HQ728350).
Serologic response was analyzed by using an in-house microimmunofluorescence assay for IgG and IgM, performed as described (4); R. conorii and R. sibirica mongolitimonae were used as antigens, and cutoff values were 1:40 for IgG and 1:20 for IgM. Acute- and convalescent-phase serum samples were obtained from 3 case-patients and single serum samples from the other 3 case-patients. Results for samples from 2 case-patients were negative, but results for the remaining 4 samples showed low to medium titers. Two samples were positive for IgM and 4 positive for IgG (Table). These results are consistent with previous reports (6), in which ≈30% of cases had a positive IgM result and ≈50% had negative or near-cutoff IgG results.
All 6 case-patients lived in Elche and its surroundings (230,112 inhabitants). Three of the cases occurred during the spring, which is when 10/18 cases reported in the literature occurred (4–14). All 6 case-patients had fever (38.5°C–39.5°C), myalgia, and headache; in the cases from the literature, 18/18 patients had fever, 13/18 myalgia, and 11/18 headache. In our study, 1 case-patient was confused and drowsy on arrival at the emergency department.
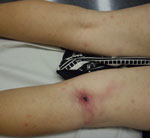
All 6 case-patients had a single inoculation eschar develop: 2 on the neck, 2 on a lower limb (Figure), 1 on the scalp, and 1 on an upper limb. Five (83%) case-patients had enlarged lymph nodes in the region from which the eschar drained, as reported for 10/18 (55%) cases from the literature. Three case-patients (50%) had lymphangitis extending from the eschar to the draining lymph nodes (Figure), compared with 6/18 (33%) in cases from the literature.
For 4/6 (67%) case-patients, a generalized maculopapular rash developed on the palms and soles but not the face; for 2 case-patients, a discrete maculopapular rash appeared after 1 day of treatment. These findings are consistent with our review of the literature, which indicated rash occurring in 13/18 (72%) cases. All 6 case-patients recovered without sequelae after antimicrobial drug treatment using doxycycline or azithromycin.
Fournier et al. (4), who reported 7 cases of R. sibirica mongolitimonae infection in 2005, proposed the name lymphangitis-associated rickettsiosis for the disease, on the basis of associated clinical features. However, for the case-patients reported here, the most common clinical signs and symptoms were fever and skin eschar, similar to those from previously reported case series; 5 of the case-patients reported here showed regional lymph node enlargement, 4 rash, and 3 lymphangitis. Because only 24 total cases have been reported and other rickettsioses produce lymphadenopathy and lymphangitis, the term lympangitis-associated rickettsiosis may be unwarranted for this disease.
In our case series, 1 case-patient had mental confusion after 10 days of a febrile disease before hospitalization and was found to be hyponatremic. In this patient, the eschar was located on the scalp, and neither rash nor other clinical clues were suggestive of rickettsiosis. The patient had increased C-reactive protein plasma levels and the highest serologic antibody titers for R. sibirica mongolitimonae of the 6 case-patients (Table, patient 1).
Since R. sibirica mongolitimonae was isolated from H. asiaticum ticks in 1991 (1), it has been recovered from H. truncatum ticks in sub-Saharan Africa (6) and H. anatolicum excavatum ticks in Greece (7). These findings suggest a possible association between R. sibirica mongolitimonae and Hyalomma spp. ticks. However, in Spain, R. sibirica mongolitimonae has been detected in 2/8 tick species tested (Rhipicephalus pusillus and Rh. bursa) at similar percentages (3.7% and 3.6%, respectively) but not from Hyalomma spp. ticks (15). Similarly, 1/20 samples of Rh. pusillus ticks from Portugal was positive for R. sibirica mongolitimonae (8), while testing of other tick species, including H. lusitanicum, Rh. sanguineus, and Rh. bursa, yielded negative results. That Hyalomma spp. (H. lusitanicum and H. marginatum) ticks have not been found to be infected in Spain does not mean that these ticks are not vectors for R. sibirica mongolitimonae. However, data from the literature support the hypothesis that Rhipicephalus spp. ticks are a vector for this rickettsia on the Iberian Peninsula, and our findings confirm that this rickettsia species is circulating in Spain, where specific vectors have yet to be described.
In summary, we report 6 cases of human infection with R. sibirica mongolitimonae that occurred in the same geographic area of Spain. Our results indicate that PCR of eschar samples is the most useful diagnostic procedure for this pathogen; samples from all 6 case-patients had positive results, while test results for 1 whole blood sample and 2 lymph node samples were negative. However, the limited number of samples does not make it possible to infer specific diagnostic sensitivities.
The epidemiology and pathogenicity of illness caused by R. sibirica mongolitimonae infection require further investigation. An active search for the vector of R. sibirica mongolitimonae in countries of the Mediterranean region is necessary to complete the epidemiology of this rickettsiosis, which is likely to be more widespread than originally assumed. In particular, clinicians caring for patients who have traveled to the Mediterranean coast of Spain should consider this rickettsiosis in the differential diagnosis.
Dr Ramos is an internist working at the Infectious Diseases Unit of Hospital General Universitario de Elche, Alicante, Spain. His research interests are emerging infectious diseases, tropical medicine, and international health.
Acknowledgment
Grant support for this work was provided by Fondo de Investigación Sanitaria PI10/00165.
References
- Yu X, Jin Y, Fan M, Xu G, Liu Q, Raoult D. Genotypic and antigenic identification of two new strains of spotted fever group rickettsiae isolated from China. J Clin Microbiol. 1993;31:83–8.PubMedGoogle Scholar
- Fournier PE, Raoult D. Current knowledge on phylogeny and taxonomy of Rickettsia spp. Ann N Y Acad Sci. 2009;1166:1–11.PubMedGoogle Scholar
- Raoult D, Brouqui P, Roux V. A new spotted-fever-group rickettsiosis. Lancet. 1996;348:412.PubMedGoogle Scholar
- Fournier PE, Tissot-Dupont H, Gallais H, Raoult DR. Rickettsia mongolotimonae: a rare pathogen in France. Emerg Infect Dis. 2000;6:290–2. DOIPubMedGoogle Scholar
- Pretorius AM, Birtles RJ. Rickettsia mongolotimonae infection in South Africa. Emerg Infect Dis. 2004;10:125–6. DOIPubMedGoogle Scholar
- Fournier PE, Gouriet F, Brouqui P, Lucht F, Raoult D. Lymphangitis-associated rickettsiosis, a new rickettsiosis caused by Rickettsia sibirica mongolotimonae: seven new cases and review of the literature. Clin Infect Dis. 2005;40:1435–44.PubMedGoogle Scholar
- Psaroulaki A, Germanakis A, Gikas A, Scoulica E, Tselentis Y. Simultaneous detection of “Rickettsia mongolotimonae” in a patient and in a tick in Greece. J Clin Microbiol. 2005;43:3558–9.PubMedGoogle Scholar
- de Sousa R, Barata C, Vitorino L, Santos-Silva M, Carrapato C, Torgal J, Rickettsia sibirica isolation from a patient and detection in ticks, Portugal. Emerg Infect Dis. 2006;12:1103–8. DOIPubMedGoogle Scholar
- de Sousa R, Duque L, Anes M, Poças J, Torgal J, Bacellar F, Lymphangitis in a Portuguese patient infected with Rickettsia sibirica. Emerg Infect Dis. 2008;14:529–30. DOIPubMedGoogle Scholar
- Caron J, Rolain JM, Mura F, Guillot B, Raoult D, Bessis D. Rickettsia sibirica subsp. mongolotimonae infection and retinal vasculitis. Emerg Infect Dis. 2008;14:683–4. DOIPubMedGoogle Scholar
- Aguirrebengoa K, Portillo A, Santibáñez S, Marín JJ, Montejo M, Oteo JA. Human Rickettsia sibirica mongolitimonae infection, Spain. Emerg Infect Dis. 2008;14:528–9. DOIPubMedGoogle Scholar
- Socolovschi C, Barbarot S, Lefebvre M, Parola P, Raoult D. Rickettsia sibirica mongolitimonae in traveler from Egypt. Emerg Infect Dis. 2010;16:1495–6. DOIPubMedGoogle Scholar
- Fleta-Asín B, Alonso-Castro L, Jado-García I, Anda-Fernández P. Detección de Rickettsia sibirica mongolotimonae en biopsia de piel de exantema mediante reacción en cadena de polimerasa: descripción de un caso. Enferm Infecc Microbiol Clin. 2011;29:778–9.PubMedGoogle Scholar
- Ibarra V, Portillo A, Palomar AM, Sanz MM, Metola L, Blanco JR, Septic shock in a patient infected with Rickettsia sibirica mongolitimonae, Spain. Clin Microbiol Infect. 2012;18:E283–5.PubMedGoogle Scholar
- Toledo A, Olmeda AS, Escudero R, Jado I, Valcárcel F, Casado-Nistal MA, Tick-borne zoonotic bacteria in ticks collected from central Spain. Am J Trop Med Hyg. 2009;81:67–74 .PubMedGoogle Scholar
Figure
Table
Cite This ArticleTable of Contents – Volume 19, Number 2—February 2013
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
José M. Ramos, Unidad de Enfermedades Infecciosas, Hospital General Universitario de Elche, Camí de la Almazara 11, 03203 Elche, Alicante, Spain
Top