Volume 19, Number 5—May 2013
Research
World Health Organization International Standard to Harmonize Assays for Detection of Hepatitis E Virus RNA
Figure 2
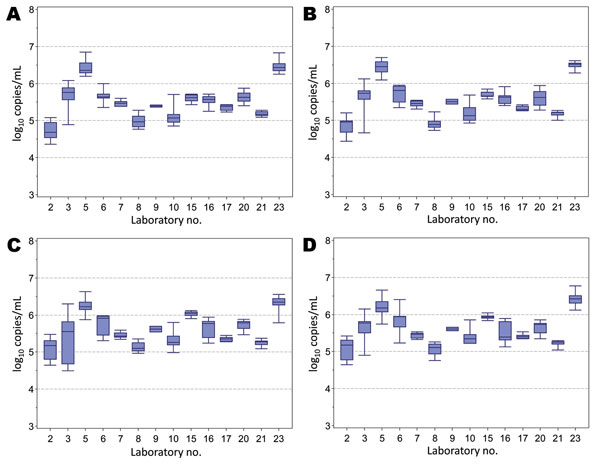
Figure 2. . . Box and whisker plots of the results for quantitative assays (log10 copies/mL) conducted by laboratories for the determination of the hepatitis E virus (HEV) RNA content of sample 1 (A), sample 2 (B), sample 3 (C), and sample 4 (D). Box indicates interquartile range; line within box indicates median; whiskers indicate minimum and maximum values observed. Laboratory code numbers are given on the horizontal axis.
1Members of the HEV Collaborative Study Group are listed at the end of this article.
2In memory of Thomas Laue.
Page created: April 01, 2013
Page updated: April 01, 2013
Page reviewed: April 01, 2013
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.