Volume 20, Number 10—October 2014
Dispatch
Human Granulocytic Anaplasmosis, South Korea, 2013
Abstract
We report a patient with human granulocytic anaplasmosis in South Korea. The patient had fever and thrombocytopenia. Human granulocytic anaplasmosis was confirmed by seroconversion, PCR, and sequence analysis for Anaplasma phagocytophilum. Morulae were observed in the cultured HL-60 cells inoculated with blood from the patient.
Anaplasma phagocytophilum, the causative agent of human granulocytic anaplasmosis (HGA), is a zoonotic tickborne pathogen transmitted by ixodid ticks that infects wild and domestic mammals and humans (1–3). HGA was first identified in the United States in 1994 (1) and subsequently in countries in Europe (3), China (4), and Japan (5).
To our knowledge, there is no report regarding the clinical description of HGA patients in South Korea. However, A. phagocytophilum has been detected in Haemaphysalis longicornis, Ixodes nipponensis, and I. persulcatus ticks (6,7) in this country. Molecular epidemiologic studies detected A. phagocytophilum in 2.6% (5/196) of striped field mice (7,8) and in 63.6% (42/66) of Korean water deer (9). Seroprevalence studies showed that 1.8% of serum samples from patients with acute fever were positive for A. phagocytophilum by an immunofluorescence antibody test (10). We report a patient with HGA and characterized the A. phagocytophilum isolate from this patient.
On May 17, 2013 (day 0, day of illness onset), fever, chills, nausea, and vomiting developed in a 57-year-old woman who lived in Chuncheon, Gangwon Province, South Korea. She came to a local clinic on the same day, and treatment with antipyretic drugs was initiated. The patient reported being bitten by a tick on her right shoulder while mountain climbing in Gangwon Province 5 days before the fever occurred. She did not recall having contact with any domestic animals and had no history of travel outside South Korea in the month before illness onset.
On day 4, the patient came to Kangwon National University Hospital (Chuncheon, South Korea) with a persistent fever despite use of antipyretics. The patient had a temperature of 39.2°C, blood pressure of 76/61 mm Hg, heart rate of 88 beats/min, and an oxygen saturation level of 98.3% on room air. An erythematous maculopapular lesion, 3 cm in diameter, was observed around the tick bite on the right shoulder. Laboratory tests showed pancytopenia and increased serum aminotransferase levels (Table).
On day 5, the patient was treated with norepinephrine, ceftriaxone, and doxycycline. On day 7, her blood pressure and temperature were within reference ranges and a norepinephrine infusion was discontinued. Peripheral blood smear showed no evidence of malaria, and antibody titer against Orientia tsutsugamushi was <1:40. Test results for antibodies against Hantaan virus, leptospira, Borrelia burgdorferi, and Coxiella burnetii were negative. Blood cultures prepared at the time of the visit were negative. On day 11, she was discharged from the hospital, and treatment with antimicrobial drugs was discontinued.
Because symptoms and laboratory findings, such as thrombocytopenia, anemia, and increased aminotransferase levels (Table), of the patient were similar to those for severe fever with thrombocytopenia syndrome (SFTS), anticoagulated blood and serum were obtained on day 5 before treatment with doxycycline and on day 19. Samples were sent to Seoul National University College of Medicine for additional diagnostic tests.
RNA was extracted from blood by using the QIAamp Viral RNA Mini Kit (QIAGEN, Hilden, Germany). Reverse transcription PCR for SFTS virus was performed according to described methods (11) and showed negative results. No cytopathic effect was observed in Vero cells and DH82 cells after inoculating them with a blood sample.
DNA was extracted from the blood by using the QIAamp DNA Mini Kit (QIAGEN) to detect A. phagocytophilum 16S rRNA gene, ankA, groESL operon, and msp2. Nested PCR was conducted to amplify a 926-bp fragment of the 16S rRNA gene by using an A. phagocytophilum species-specific primer set (12). Direct sequencing of the PCR product was performed to confirm A. phagocytophilum, and the partial 16S rRNA gene sequence was deposited in GenBank (accession no. KF805344).
A nucleotide basic local alignment search tool (BLAST) search (http://blast.ncbi.nlm.nih.gov/Blast.cgi) with the obtained sequence showed matches to only A. phagocytophilum sequences (99.3%–100% similarities). Aligning ≥2 sequences by using BLAST showed that the obtained sequence had lower similarities (94.3%–98.6%) with other Anaplasma species (A. marginale [GenBank accession no. NR_074556], A. centrale [NR_074356], A. ovis [AY262124], A. platys [EF139459], and A. bovis [HM131218]) and with Ehrlichia species (E. chaffeensis [NR_037059], E. ewingii [NR_044747], and E. canis [NR_074386]).
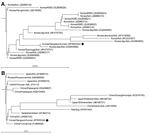
Comparison of the 16S rRNA gene sequence with sequences for other A. phagocytophilum strains from South Korea showed that this sequence was relatively distant from those of Korean water deer isolates, but similar to those of tick isolates from Jeju Island (Figure 1, panel A). Comparison of the 16S rRNA gene sequence with other sequences of A. phagocytophilum strains reported from other countries showed that our isolate was relatively distant from the strains obtained from ticks collected in Gansu and Guangxi, China, and Hokkaido and Shimane, Japan. The isolate was similar to a human isolate from the United States, tick isolates from Russia and China, and animal isolates from Yunnan, Zhejiang, and Hubei, China (Figure 1, panel B).
Nested PCR was performed to amplify a 667-bp fragment of the ankA gene (13), and single non-nested PCRs were performed to amplify a 1,715-bp fragment of the groESL operon (14) and a 334-bp fragment of the msp2 gene (15). Direct sequencing of PCR products was performed to confirm A. phagocytophilum, and partial ankA, groESL, and msp2 sequences were deposited in GenBank (accession nos.KJ677106–KJ677108). Nucleotide BLAST searches with obtained sequences showed matches to only A. phagocytophilum sequences: 94.1%–97.9% similarities for ankA, 97.9%–99.5% for groESL, and 97.8%–99.6% for msp2.
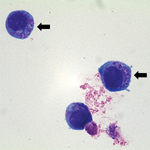
For A. phagocytophilum culturing, a suspension of human promyelocytic cell line HL-60 (ATCC CCL-240) was inoculated with the patient’s blood sample (day 5 postillness, before treatment with antimicrobial drugs) and incubated at 37°C in an atmosphere of 5% CO2 (10). The culture suspension was examined microscopically by Wright-Giemsa staining of cytocentrifuged preparations of cells at 2 to 3-day intervals. Subculture was performed on day 14 postinoculation. On day 8 post-subculture, morulae within cultured HL-60 cells were observed (Figure 2).
Nested PCRs to amplify 16S rRNA and ankA genes showed positive results for isolates from day 14 postinoculation and from day 10 postsubculture. Single, non-nested PCRs to amplify groESL and msp2 genes showed positive results for the isolate from day 14 postinoculation but negative for the isolate from day 10 postsubculture. Comparison of sequences from culture isolates and those from PCR products directly amplified from the blood sample showed that these sequences were identical.
For serologic diagnosis, an immunofluorescence antibody test kit for A. phagocytophilum (Fuller Laboratories, Fullerton, CA, USA) was used. Positive cutoff titers were 1:16 for IgM and 1:80 for IgG, according to the manufacturer’s instructions. Specific IgM titers increased from 1:16 (day 5) to ≥1:256 (day 19). Specific IgG antibody titers increased from 1:20 (day 5) to 1:160 (day 19).
We confirmed a case of HGA in a patient in South Korea (who was suspected of having SFTS) by using serologic analysis, PCR, culture, and sequence analysis for A. phagocytophilum. According to the case definition of HGA of the US Centers for Disease Control and Prevention (Atlanta, GA, USA) (2), this case fulfilled the criteria for anaplasmosis. The patient had a history of a tick bite, and the clinical symptoms and laboratory findings were similar to those of SFTS, an emerging vector-borne disease in South Korea (11).
No effective treatment for SFTS is available, but doxycycline is effective for treating HGA (2). Clinical features of HGA may be confused with those for SFTS, which may result in inappropriate treatment and severe outcomes. Therefore, not only SFTS but also HGA should be considered as differential diagnoses for patients with fevers and thrombocytopenia after tick bites.
Dr Kye-Hyung Kim is an infectious disease physician and a senior researcher at Seoul National University College of Medicine, Seoul, South Korea. Her primary research interests are medical virology and emerging infectious diseases.
References
- Chen SM, Dumler JS, Bakken JS, Walker DH. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–95 .PubMedGoogle Scholar
- Chapman AS, Bakken JS, Folk SM, Paddock CD, Bloch KC, Krusell A, Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis—United States: a practical guide for physicians and other health-care and public health professionals. MMWR Recomm Rep. 2006;55:1–27 .PubMedGoogle Scholar
- Petrovec M, Lotric Furlan S, Zupanc TA, Strle F, Brouqui P, Roux V, Human disease in Europe caused by a granulocytic Ehrlichia species. J Clin Microbiol. 1997;35:1556–9 .PubMedGoogle Scholar
- Zhang LC, Wang L, Zhang L, Zhang J, Yang S, Han J. Anaplasma phagocytophilum and Ehrlichia chaffeensis in Yiyuan County, Shandong. Infectious Disease Information. 2009;1:21–5.
- Ohashi N. Gaowa, Wuritu, Kawamori F, Wu D, Yoshikawa Y, et al. Human granulocytic anaplasmosis, Japan. Emerg Infect Dis. 2013;19:289–92.
- Kim CM, Kim MS, Park MS, Park JH, Chae JS. Identification of Ehrlichia chaffeensis, Anaplasma phagocytophilum, and A. bovis in Haemaphysalis longicornis and Ixodes persulcatus ticks from Korea. Vector Borne Zoonotic Dis. 2003;3:17–26. DOIPubMedGoogle Scholar
- Chae JS. Yu do H, Shringi S, Klein TA, Kim HC, Chong ST, et al. Microbial pathogens in ticks, rodents and a shrew in northern Gyeonggi-do near the DMZ, Korea. J Vet Sci. 2008;9:285–93.
- Chae JS, Kim CM, Kim EH, Hur EJ, Klein TA, Kang TK, Molecular epidemiological study for tick-borne disease (Ehrlichia and Anaplasma spp.) surveillance at selected U.S. military training sites/installations in Korea. Ann N Y Acad Sci. 2003;990:118–25. DOIPubMedGoogle Scholar
- Kang JG, Ko S, Kim YJ, Yang HJ, Lee H, Shin NS, New genetic variants of Anaplasma phagocytophilum and Anaplasma bovis from Korean water deer (Hydropotes inermis argyropus). Vector Borne Zoonotic Dis. 2011;11:929–38. DOIPubMedGoogle Scholar
- Heo EJ, Park JH, Koo JR, Park MS, Park MY, Dumler JS, Serologic and molecular detection of Ehrlichia chaffeensis and Anaplasma phagocytophila (human granulocytic ehrlichiosis agent) in Korean patients. J Clin Microbiol. 2002;40:3082–5. DOIPubMedGoogle Scholar
- Kim K-H, Yi J, Kim G, Choi SJ, Jun KI, Kim N-H, Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerg Infect Dis. 2013;19:1892–4. DOIPubMedGoogle Scholar
- Oh JY, Moon B-C, Bae BK, Shin E-H, Ko YH, Kim Y-J, Genetic identification and phylogenetic analysis of Anaplasma and Ehrlichia species in Haemaphysalis longicornis collected from Jeju Island, Korea. Journal of Bacteriology and Virology. 2009;39:257–67. DOIGoogle Scholar
- Massung RF, Levin ML, Munderloh UG, Silverman DJ, Lynch MJ, Gaywee JK, Isolation and propagation of the Ap-Variant 1 strain of Anaplasma phagocytophilum in a tick cell line. J Clin Microbiol. 2007;45:2138–43. DOIPubMedGoogle Scholar
- Chae JS, Foley JE, Dumler JS, Madigan JE. Comparison of the nucleotide sequences of 16S rRNA, 444 Ep-ank, and groESL heat shock operon genes in naturally occurring Ehrlichia equi and human granulocytic ehrlichiosis agent isolates from northern California. J Clin Microbiol. 2000;38:1364–9 .PubMedGoogle Scholar
- Levin ML, Nicholson WL, Massung RF, Sumner JW, Fish D. Comparison of the reservoir competence of medium-sized mammals and Peromyscus leucopus for Anaplasma phagocytophilum in Connecticut. Vector Borne Zoonotic Dis. 2002;2:125–36. DOIPubMedGoogle Scholar
Figures
Table
Cite This Article1These authors contributed equally to this article.
Table of Contents – Volume 20, Number 10—October 2014
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Myoung-don Oh, Department of Internal Medicine, Seoul National University College of Medicine, 103 Daehak-ro, Jongno-gu, Seoul 110-744, South Korea
Top