Volume 20, Number 12—December 2014
Dispatch
Zoonotic Bartonella Species in Cardiac Valves of Healthy Coyotes, California, USA
Cite This Article
Citation for Media
Bartonellae are vector-borne gram-negative, aerobic, intracellular bacteria with a tropism for erythrocytes and endothelial cells (1). These bacteria, many of which are zoonotic, infect a wide range of domestic and wild animal species, causing a spectrum of disease manifestations and pathologies (2). Bartonellae, especially Bartonella vinsonii subsp. berkhoffii (B. v. berkhoffii), cause valvular endocarditis, especially of the aortic valve in mammals, including humans, dogs, cats, and cattle (1,3). Fleas and possibly ticks can vector B. v. berkhoffii (4). Bartonella species, typically observed in 5- to 7-year-old mid-sized to large dogs, account for ≈28% of endocarditis in dogs (3,5). Bartonellae, including B. v. berkhoffii, account for ≈3% of human endocarditis cases (1,6). In dogs and humans, these bacteria appear to have a specific tropism for aortic and mitral valves (1). Similar to lesions that develop with Coxiella burnetii endocarditis (7), valvular vegetative lesions can result from chronic Bartonella infection.
In California, coyotes (Canis latrans) are a major reservoir for B. v. berkhoffii (8). Natural Bartonella reservoir hosts are often asymptomatic, but to our knowledge, the possible role of Bartonella-induced endocarditis in coyotes has never been investigated. We hypothesized that B. v. berkhoffii or other Bartonella species could cause endocarditis in coyotes. We also hypothesized that bartonellae might preferentially localize to the aortic and/or mitral valves before vegetative lesions develop. Hence, coyotes served as a naturally occurring epidemiologic and physiologic sentinel model for studying infection kinetics and pathology induced by this bacterium in a reservoir host (coyotes).
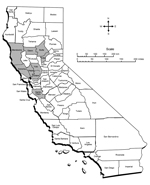
During the early 2000s, a total of 70 coyotes trapped in 9 northern California counties as part of a depredation control program were assessed for zoonotic heartworm (Dirofilaria immitis) disease (9). Coyote hearts and spleens were collected at that time and stored at –70°C in a manner to avoid DNA carryover during handling, storage, and processing. In 2012 and 2013, the hearts were dissected for macroscopic evidence of aortic and mitral valve vegetative endocarditis lesions. A board-certified veterinary pathologist examined possible valvular lesions or thickened regions; however, because the samples had been frozen, microscopic histopathologic examination was not conducted. We extracted DNA from aortic and mitral valves and spleens using DNeasy Blood and Tissue Kits (QIAGEN, Hilden, Germany). B. v. berkhoffii–spiked rabbit blood was the DNA extraction positive control. We tested extracted samples by PCR for Bartonella DNA targeting the citrate synthase gene (gltA) (10). PCR of spleen tissue was a substitute for blood culture detection of bacteremia. B. henselae DNA and distilled water were PCR-positive and -negative controls, respectively. Partial gene sequencing was performed on PCR-positive tissues. Nineteen aortic valve, mitral valve, and splenic DNA samples from 14 coyotes (B. v. berkhoffii PCR-positive animals by gltA PCR and sequencing) were genotyped by using primers targeting 16–23S intergenic transcribed spacer (ITS) region, as previously described (11) with minor modifications in annealing temperature (68°C for 15 s) and extension (72°C for 18 s). We conducted statistical analysis for differences in tissue tropism using Epi Info version 6 (Centers for Disease Control and Prevention, Atlanta, GA, USA).
Of the 70 coyotes collected from 9 counties (Figure), 45 (64%) were male. Coyotes’ ages ranged from <1 year (57 [81%]) to >5 years (3 [4%]). Nine (20%) male and 6 (24%) female coyotes were PCR positive for Bartonella species. Fourteen (93%) of the 15 Bartonella-positive coyotes were <3 years old, of which 13 (87%) were <1 year old. Prevalence by county ranged from 0% to 33% (Figure).
We found no gross vegetative aortic or mitral valvular endocarditis lesions. Fifteen (21%) coyotes tested positive by PCR for Bartonella gltA gene. Overall, 8 aortic valves, 5 mitral valves, and 4 spleens were PCR positive. Aortic and mitral cardiac valves of 1 coyote (no. 93) tested positive by PCR for B. v. berkhoffii, and the aortic valve and spleen of another coyote (no. 110) were PCR positive (Table). Although a higher percentage of positive cardiac valves were aortic (53%) than mitral (33%), the difference was not significant. However, when we compared the number of Bartonella-infected cardiac valves (11 valves) with Bartonella-infected spleens (3 spleens), we found that Bartonella DNA was amplified 4.16 times (95% CI 1.02–24.12) more often from cardiac valves than from spleens.
Partial DNA sequencing showed that aortic valves from 8 (53%) of 15 coyotes were B. v. berkhoffii positive, compared with mitral valves from 4 (27%) and spleens from 3 (20%) coyotes. B. rochalimae was amplified from the spleen of coyote no. 99, and B. henselae DNA was amplified from the mitral valve of coyote no. 137 (Table). Of 14 coyotes tested for B. v. berkhoffii genotypes by 16–23S ITS PCR, 8 were positive, whereas Bartonella DNA was not amplified from the remaining 6 tissue DNA samples by using ITS primers. By sequence analyses, 4 coyotes were infected with B. v. berkhoffii genotype I, 3 with genotype II, and 1 with genotype III.
Our study documents the presence of 3 zoonotic Bartonella species in heart valves and/or spleen of free-ranging coyotes from northern California. Despite the absence of gross vegetative endocardial lesions, Bartonella DNA was amplified and sequenced from >20% of the coyotes, mainly from cardiac valves; only 4 (6%) coyotes had PCR-positive spleens, compared with 12 (17%) coyotes with PCR-positive cardiac valves. We hypothesize that Bartonella in the spleen indicated early or ongoing bacteremia, whereas bartonellae in the heart valves, in their absence in the spleen, indicated valvular bacterial localization, possibly facilitating persistent infection that could evolve through time to endocarditis. This evolution has been observed for C. burnetii infection in humans (12), for which the mean reported interval from illness onset to endocarditis diagnosis is 12–24 months (7). Bartonella endocarditis is usually seen in middle-aged dogs (mean age 6.3 years ± 2.8) (3,5) and in adult humans (mean age 54 years ± 16) (6). Because 93% of the PCR-positive coyotes were <3 years old, they were very likely too young for vegetative endocarditis to have developed.
Nevertheless, the fact that ≈20% of the cardiac valve tissues were PCR positive for Bartonella perhaps indicates that the bacteria had localized to the valves of infected coyotes. B. v. berkhoffii can induce vasoproliferative lesions in animals (13); thus, cases of Bartonella endocarditis might represent only a small fraction of infected animals that have chronic cardiac valvular localization. All 3 B. v. berkhoffii genotypes identified in these coyotes have been previously involved in humans or dogs with endocarditis (14,15). To our knowledge, B. henselae and B. v. berkhoffii genotype III have not been previously identified in coyotes; thus, these mammals can be added to the list of susceptible species. Coyotes might be a natural reservoir for B. v. berkhoffii genotype III, which so far has been mainly described in California gray foxes (14).
In conclusion, Bartonella infection of a natural reservoir appears to lead to cardiac valve tropism. This tropism could result in development of endocarditis, a severe and often lethal complication of Bartonella infection.
Mr Kehoe is a fourth-year veterinary student at the University of California, Davis. His primary research interests include zoologic and wildlife medicine and health and emerging zoonotic infections.
Acknowledgment
We investigated whether Bartonella spp. could cause endocarditis in coyotes or localize to cardiac valves before lesions develop. Bartonella DNA was amplified more often from coyote cardiac valves than spleen. Bartonella infection apparently leads to cardiac valve tropism, which could cause endocarditis, an often lethal complication in mammals, including humans.
References
- Chomel BB, Kasten RW, Williams C, Wey AC, Henn JB, Maggi R, Bartonella endocarditis: a pathology shared by animal reservoirs and patients. Ann N Y Acad Sci. 2009;1166:120–6 . DOIPubMedGoogle Scholar
- Boulouis HJ, Chang CC, Henn JB, Kasten RW, Chomel BB. Factors associated with the rapid emergence of zoonotic Bartonella infections. Vet Res. 2005;36:383–410. DOIPubMedGoogle Scholar
- MacDonald KA, Chomel BB, Kittleson MD, Kasten RW, Thomas WP, Pesavento P. A prospective study of canine infective endocarditis in northern California (1999–2001): emergence of Bartonella as a prevalent etiologic agent. J Vet Intern Med. 2004;18:56–64 .PubMedGoogle Scholar
- Yore K, Digangi B, Brewer M, Balakrishnan N, Breitschwerdt EB, Lappin M. Flea species infesting dogs in Florida and Bartonella spp. prevalence rates. Vet Parasitol. 2014;199:225–9 . DOIPubMedGoogle Scholar
- Sykes JE, Kittleson MD, Pesavento PA, Byrne BA, MacDonald KA, Chomel BB. Evaluation of the relationship between causative organisms and clinical characteristics of infective endocarditis in dogs: 71 cases (1992–2005). J Am Vet Med Assoc. 2006;228:1723–34. DOIPubMedGoogle Scholar
- Houpikian P, Raoult D. Diagnostic methods. Current best practices and guidelines for identification of difficult-to-culture pathogens in infective endocarditis. Cardiol Clin. 2003;21:207–17. DOIPubMedGoogle Scholar
- Stein A, Raoult D, Q fever endocarditis. Eur Heart J. 1995;16 Suppl B:19.
- Chang CC, Kasten RW, Chomel BB, Simpson DC, Hew CM, Kordick DL, Coyotes (Canis latrans) as the reservoir for a human pathogenic Bartonella sp.: molecular epidemiology of Bartonella vinsonii subsp. berkhoffii infection in coyotes from central coastal California. J Clin Microbiol. 2000;38:4193–200 .PubMedGoogle Scholar
- Sacks BN, Chomel BB, Kasten RW, Chang CC, Sanders RK, Leterme SD. Validation for use with coyotes (Canis latrans) of a commercially available enzyme-linked immunosorbent assay for Dirofilaria immitis. Vet Parasitol. 2002;109:45–58. DOIPubMedGoogle Scholar
- Norman AF, Regnery R, Jameson P, Greene C, Krause DC. Differentiation of Bartonella-like isolates at the species level by PCR-restriction fragment length polymorphism in the citrate synthase gene. J Clin Microbiol. 1995;33:1797–803 .PubMedGoogle Scholar
- Varanat M, Maggi RG, Linder KE, Breitschwerdt EB. Molecular prevalence of Bartonella, Babesia, and hemotropic Mycoplasma sp. in dogs with splenic disease. J Vet Intern Med. 2011;25:1284–91. DOIPubMedGoogle Scholar
- Fenollar F, Fournier PE, Carrieri MP, Habib G, Messana T, Raoult D. Risks factors and prevention of Q fever endocarditis. Clin Infect Dis. 2001;33:312–6. DOIPubMedGoogle Scholar
- Beerlage C, Varanat M, Linder K, Maggi RG, Cooley J, Kempf VA, Bartonella vinsonii subsp. berkhoffii and Bartonella henselae as potential causes of proliferative vascular diseases in animals. Med Microbiol Immunol (Berl). 2012;201:319–26. DOIPubMedGoogle Scholar
- Maggi RG, Chomel B, Hegarty BC, Henn J, Breitschwerdt EB. A Bartonella vinsonii berkhoffii typing scheme based upon 16S–23S ITS and Pap31 sequences from dog, coyote, gray fox, and human isolates. Mol Cell Probes. 2006;20:128–34 . DOIPubMedGoogle Scholar
- Cadenas MB, Bradley J, Maggi RG, Takara M, Hegarty BC, Breitschwerdt EB. Molecular characterization of Bartonella vinsonii subsp. berkhoffii genotype III. J Clin Microbiol. 2008;46:1858–60 . DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleTable of Contents – Volume 20, Number 12—December 2014
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Bruno B. Chomel, Department of Population Health and Reproduction, School of Veterinary Medicine, VM3B, 1089 Veterinary Medicine Dr, University of California, Davis, Davis, CA 95616, USA
Top