Volume 20, Number 12—December 2014
Letter
Molecular Characterization of Borrelia burgdorferi from Case of Autochthonous Lyme Arthritis
To the Editor: The first Lyme borreliosis (LB) case reported to be acquired in California occurred in 1978 (1). During the past 10 years, 744 confirmed LB cases were reported in California; 419 (56.2%) were likely acquired in-state. The highest incidence of this disease occurs in northern coastal California, in locations such as Santa Cruz County (2), where habitat supports yearlong activity of the tick vector Ixodes pacificus (3,4).
Existing data describe the genetic diversity of the LB agent Borrelia burgdorferi among ticks in Californa (5,6), but few instances of direct detection and genetic characterization of B. burgdorferi sensu stricto in samples from humans are documented in California. B. burgdorferi has been isolated from skin biopsy samples of 3 patients in California in whom LB was diagnosed (1). Seinost et al. genotyped strains isolated in the United States, including 7 isolates identified in California from skin, blood, or cerebrospinal fluid, but no documented exposure information was available (7). Girard et al. genotyped B. burgdorferi in 10- to 12-year-old stored serum samples collected from 22 northern California residents, some of whom were asymptomatic at time of collection. Of 22 PCR-positive specimens, 21 had the single laboratory type strain B31 genotype (3).
A 12-year-old resident of Santa Cruz County, California, came to the emergency department of Dominican Hospital in September 2012 with a swollen, painful right knee and mildly painful right hip. The patient’s family reported that LB had been diagnosed by a local physician. Illness onset was in May 2010; symptoms consisted of recurrent knee swelling and pain lasting several days every 4–5 months and positive serologic test results for B. burdorferi (not available). The patient had not traveled outside of California during the preceding 6 years. In May 2011, an IgG Western blot of the patient’s serum that was processed at a commercial laboratory showed immunoreactive bands of 18, 23, 28, 30, 39, 41, 45, 58, 66, and 93 kDa. In both 2010 and 2011, the patient’s family had chosen to give the patient unspecified herbal treatments instead of antibacterial drugs.
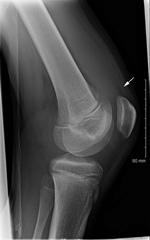
On physical examination in the emergency department, the patient’s right knee was swollen; knee flexion was reduced to 30°. The right hip was painful on rotation. Serum laboratory values included a leukocyte count of 7,000/μL, hematocrit 33%, and erythrocyte sedimentation rate of 73mm/h. Plain radiograph images of the right hip did not show any abnormalities; the radiograph of the right knee showed suprapatellar effusion (Figure). Right knee aspiration yielded 115 mL of cloudy yellow fluid; laboratory tests showed a leukocyte count of 59,750/μL and protein level 5 g/dL; no crystals were noted. Results of routine bacterial culture of synovial fluid were negative. Amoxicillin was prescribed for a suspected septic joint and was taken for 1 week. Nine months later, the patient was reportedly asymptomatic and had returned to normal activity.
Right knee synovial fluid was sent to ARUP Laboratories (Salt Lake City, UT, USA); results were positive for the B. burgdorferi sensu lato recA gene by use of a proprietary qualitative PCR procedure. At the University of California, Irvine, we thawed another aliquot of synovial fluid, which had been frozen without cryoprotectant, and inoculated samples into BSK II medium (8). After incubation for 2 weeks at 34°C, no spirochetes were noted. We subjected another 100 μL aliquot to DNA extraction using DNeasy Blood and Tissue Kit and the QIAcube apparatus (QIAGEN, Valencia, CA, USA). We used multiplex quantitative PCR (qPCR) and primers and specific probes for the 16S ribosomal RNA genes of LB group species and for relapsing fever group species of Borrelia in 2 replicates as described by Barbour et al. (9). By qPCR, there were 18 gene copies of an LB group species in 1 replicate and 23 copies in the other. The qPCR results for relapsing fever group species, including B. miyamotoi and B. hermsii, which are enzootic in parts of California, were negative. We genotyped the ospC allele and 16S–23S intergenic spacer (IGS) using PCR amplification of each locus and direct sequencing as described by Travinsky et al. (6). Sequencing of the targeted PCR products showed that the ospC allele was type Hb and the IGS genotype was 13.
Two years of untreated relapsing pauciarticular arthritis of the knee and hip, a B. burgdorferi–positive Western blot, and laboratory detection of B. burgdorferi from synovial fluid by PCR in 2 different laboratories leads us to conclude that the patient had Lyme arthritis. This patient likely acquired the infection locally. The prevalence of B. burgdorferi in nymphal I. pacificus ticks (range 4%–10%) in Santa Cruz County, and >10% of the geographic area of the county is categorized as being at high acarologic risk for LB (4). To our knowledge, the combination of ospC allele Hb and IGS genotype 13 has been identified only in California to date (6,8). A type “H” ospC type was reported from synovial fluid from LB patients from the eastern United States (10), but in the absence of IGS determination, this was probably type Ha, which is more typical of that region (8). The addition of the IGS locus to ospC alleles provides a precise approach to characterize genetic diversity and potential origin of B. burgdorferi in human tissue.
Acknowledgments
We thank the staff of Dominican Hospital, Santa Cruz, California, for facilitating access to case information; the staff of the County of Santa Cruz communicable disease division for their continued support; and California Epidemiologic Investigation Services for their suggestions for this manuscript and the work of their fellow, Sharon I. Brummitt.
The project described was supported by the Preventive Health Services Block Grant from the Centers for Disease Control and Prevention. Laboratory work at University of California Irvine was supported by the National Institutes of Health grant AI-065359. This work was determined exempt by The Committee for the Protection of Human Subjects, California Office of State Health and Planning.
References
- Fritz CL, Vugia DJ. Clinical issues in Lyme borreliosis: a California perspective. Infectious Disease Review. 2001;3:111–22.
- California Department of Public Health, Vector-Borne Disease Section. California: information for health professionals: tick-borne disease of interest in California (updated 2014 Mar 10; cited 2014 Mar 11). http://www.cdph.ca.gov/HealthInfo/discond/Documents/EpiandPreventionofTBDInterestinCa.ppsx
- Girard YA, Fedorova N, Lane RS. Genetic diversity of Borrelia burgdorferi and detection of B. bissettii-like DNA in serum of north-coastal California residents. J Clin Microbiol. 2011;49:945–54. DOIPubMedGoogle Scholar
- Lane RS, Manweiler SA, Stubbs HA, Lennette ET, Madigan JE, Lavoie PE. Risk factors for Lyme disease in a small rural community in northern California. Am J Epidemiol. 1992;136:1358–68 .PubMedGoogle Scholar
- Girard YA, Travinsky B, Schotthoefer A, Fedorova N, Eisen RJ, Eisen L, Population structure of the Lyme borreliosis spirochete Borrelia burgdorferi in the Western black-legged tick (Ixodes pacificus) in northern California. Appl Environ Microbiol. 2009;75:7243–52. DOIPubMedGoogle Scholar
- Travinsky B, Bunikis J, Barbour AG. Geographic differences in genetic locus linkages for Borrelia burgdorferi. Emerg Infect Dis. 2010;16:1147–50. DOIPubMedGoogle Scholar
- Seinost G, Dykhuizen DE, Dattwyler RJ, Golde WT, Dunn JJ, Wang IN, Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect Immun. 1999;67:3518–24 .PubMedGoogle Scholar
- Barbour AG, Travinsky B. Evolution and distribution of the ospC gene, a transferable serotype determinant of Borrelia burgdorferi. MBio. 2010;1: .DOIPubMedGoogle Scholar
- Barbour AG, Bunikis J, Travinsky B, Gatewood HA, Diuk-Wasser M, Fish D, Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same reservoir host and arthropod vector. Am J Trop Med Hyg. 2009;81:1120–31. DOIPubMedGoogle Scholar
- Strle K, Jones KL, Drouin EE, Li X, Steere AC. Borrelia burgdorferi RST 1 (OspC Type A) Genotype is associated with greater inflammation and more severe Lyme disease. Am J Pathol. 2011;178:2726–39. DOIPubMedGoogle Scholar
Figure
Cite This ArticleRelated Links
Table of Contents – Volume 20, Number 12—December 2014
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Anne M. Kjemtrup, California Department of Public Health, 1616 Capitol Ave, MS 7307, PO Box 997377, Sacramento, CA 95899-9730. USA
Top