Volume 20, Number 2—February 2014
Research
Novel Paramyxovirus Associated with Severe Acute Febrile Disease, South Sudan and Uganda, 2012
Abstract
In 2012, a female wildlife biologist experienced fever, malaise, headache, generalized myalgia and arthralgia, neck stiffness, and a sore throat shortly after returning to the United States from a 6-week field expedition to South Sudan and Uganda. She was hospitalized, after which a maculopapular rash developed and became confluent. When the patient was discharged from the hospital on day 14, arthralgia and myalgia had improved, oropharynx ulcerations had healed, the rash had resolved without desquamation, and blood counts and hepatic enzyme levels were returning to reference levels. After several known suspect pathogens were ruled out as the cause of her illness, deep sequencing and metagenomics analysis revealed a novel paramyxovirus related to rubula-like viruses isolated from fruit bats.
Paramyxoviruses comprise a large family of viruses, including pathogens that cause severe disease in humans (1). Worldwide, >100 paramyxoviruses have been identified in bats and rodents (2–4). Among these, few have been shown to be pathogenic to humans, possibly because of limited host range and/or infrequent exposure. We describe a novel rubula-like virus that was associated with a severe acute febrile illness in a woman. The patient was a wildlife biologist who had participated in a 6-week field expedition to South Sudan and Uganda. During this expedition, she had been exposed to bats and rodents of >20 species while wearing different levels of personal protective equipment.
During the summer of 2012, a 25-year-old female wildlife biologist participated in a 6-week field expedition to South Sudan and Uganda, where she traveled to remote rural areas collecting bats and rodents for ecologic research. In the course of her duties, she manipulated animals in traps and mist nets, performed dissections, collected blood and tissues, and visited caves with large bat populations. She received no injuries from sharp objects and no bites or scratches from the animals with which she was working. She occasionally used respiratory protection when working with animals and specimens, and she wore a respirator while in caves. During her trip, she had no known contact with ill members of the field team, no contact with health care facilities, and no sexual contacts. She had been vaccinated against hepatitis A and B, yellow fever, measles, mumps, rubella, rabies, polio, tetanus/diphtheria, and typhoid fever, and she fully complied with a malaria prophylaxis regimen of atovaquone/proguanil. Her medical history included migraines and treatment of presumptive malaria with artemether/lumefantrine during a similar expedition the previous year.
Five days after returning to the United States, the woman was evaluated in the emergency department for a 2-day history of fever, malaise, headache, generalized myalgia and arthralgia, neck stiffness, a metallic taste, and sore throat. Results of rapid malaria test, performed on the day of fever onset, were negative. Other laboratory results and the patient’s vital signs at the time of admission are summarized in the Table. The patient seemed to be fatigued but alert and oriented; she was anicteric, and she had no nuchal rigidity or focal neurologic deficits. Mild erythema of the soft palate without ulcerations or exudates was noted. The spleen tip was palpable despite absence of adenopathy.
Examination of heart, abdomen, and lungs (including chest radiographs) revealed no abnormalities. No rash or synovitis was noted. Treatment with intravenous ceftriaxone was begun for possible typhoid fever, and artemether/lumefantrine was continued for presumptive malaria.
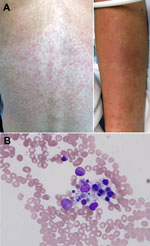
On hospital day 2, a maculopapular rash erupted over the patient’s trunk (Figure 1, panel A), several small aphthous ulcers appeared on her soft palate, and she had mild diarrhea. As long as the fever persisted, clear pulse/temperature dissociation was present (positive Faget sign); however, hemodynamics, oxygenation, and renal function were stable. Doxycycline was added for the expanded differential diagnosis of a rickettsial illness or plague. On hospital day 3, fever, headache, and myalgia persisted, and the patient experienced bloody emesis, mild diarrhea positive for occult blood but without frank hematochezia or melena, and minimal diffuse abdominal tenderness. Her menstrual period occurred without substantial menorrhagia. The rash became confluent; a centrifugal distribution and prominent petechia appeared at sites of trauma or pressure.
The possibility of hemophagocytosis was considered, and a bone marrow biopsy sample was obtained on day 4. The sample showed a mild increase in macrocytic hemophagocytosis and pancytopenia with a hypocellular marrow with myeloid hyperplasia and erythroid hypoplasia (Figure 1, panel B).
The fever slowly but progressively decreased, and the patient improved over the next few days; the last recorded fever was on hospital day 9. Abdominal pain and diarrhea resolved. Ceftriaxone was discontinued after 8 days. When the patient was discharged on hospital day 14, arthralgia and myalgia had improved, oropharynx ulcerations had healed, the rash had resolved without desquamation, and blood counts and hepatic enzyme levels were returning to reference limits. Considerable sequelae (myalgia, arthralgia, headache, malaise, and fatigue) persisted for several months.
The initial suspected diagnosis was hematophagocytic syndrome (hemophagocytic lymphohistiocytosis). This clinical syndrome has been associated with a variety of viral, bacterial, fungal, and parasitic infections, as well as collagen–vascular diseases and malignancies. Initial diagnostic testing for various infectious diseases included blood screening for respiratory viruses, HIV, cytomegalovirus, and malaria parasites; all results were negative.
On hospital day 2, a diagnosis of a viral hemorrhagic fever was considered, and blood specimens were sent to the Centers for Disease Control and Prevention (CDC) for testing. Molecular testing results were negative for rickettsiae, filoviruses (Marburgviruses and Ebolaviruses), selected bunyaviruses (Rift Valley fever virus, Crimean Congo hemorrhagic fever virus), arenaviruses (Lassa, Lujo, and lymphocytic choriomeningitis viruses), and several arboviruses (yellow fever, dengue, O'nyong-nyong, chikungunya, and Zika viruses).
A pathogen-discovery deep-sequencing protocol was followed, as described (5,6). In brief, total RNA was extracted from blood and serum samples obtained 3 days after symptom onset; RNA was nonspecifically amplified with previously described primers (7). The cDNA library was sequenced on a 454 FLX Genome Sequencer (Roche Diagnostics, Indianapolis, IN, USA). Unassembled sequences were translated and compared with the nonredundant protein database from the National Center for Biotechnology Information by using a BLASTx algorithm (www.ncbi.nlm.nih.gov/blast/Blast.cgi). The sequence reads linked to the BLASTx results were distributed into taxa by using MEGAN (8). Metagenomic analysis revealed a novel paramyxovirus in the patient’s blood and serum; the virus was most closely related to Tuhoko virus 3, a rubula-like virus (family Paramyxoviridae) recently isolated from Rousettus leschenaultii fruit bats in southern China (4). The next-generation sequence reads with homology to Tuhoko virus 3 covered ≈25% of the expected complete virus genome. Based on the sequences obtained, a series of primers were designed to amplify overlapping fragments spanning the complete genome of this novel virus. A detailed list of primers is available upon request. Amplicons of different sizes were obtained by standard reverse transcription PCR (RT-PCR) and sequenced by the standard Sanger method (5,6).
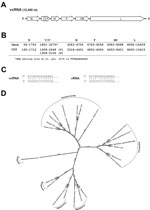
This novel paramyxovirus is provisionally named Sosuga virus in recognition of its probable geographic origin (South Sudan, Uganda). The complete genome of Sosuga virus was 15,480 nt long and conformed to the paramyxovirus rule of 6 (1). The genome organization (Figure 2, panels A, B) resembled that of most paramyxoviruses, containing 6 genes, N, V/P, M, F, HN, and L, encoding the 7 viral proteins: nucleocapsid (N), V protein (V), phosphoprotein (P), matrix protein (M), fusion protein (F), hemagglutinin–neuraminidase (HN), and polymerase (L). The sequence of the RNA editing site in the V/P gene is identical to that of Tuhoko virus 3 (4). The faithful transcription of V/P generates the V mRNA, and a GG insertion at the editing site generates the P mRNA. In addition, the terminal 5′ and 3′ sequences of the virus were experimentally determined (Figure 2, panel C) by rapid amplification of cDNA ends as described (9).
Pairwise comparison of the full-length sequence of Sosuga virus with the closest related viruses showed 61.6%, 53.1%, and 51.4% identities, respectively, with Tuhoko virus 3, Achimota virus 1, and Achimota virus 2 (Achimota viruses were isolated from the Eidolon helvum fruit bat in Ghana) (3). When the deduced amino acid sequences of Sosuga virus were compared with those of Tuhoko virus, 3 proteins revealed overall amino acid identities ranging from 57.4% (HN) to 84% (N). Phylogenetic analysis of Sosuga virus and other paramyxoviruses clearly showed that Sosuga virus clusters with other bat-borne rubula-like viruses, which are closely related to rubulaviruses but have not yet been classified as such (Figure 2, panel D).
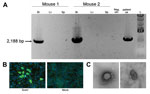
Virus isolation was attempted by inoculating monolayers of Vero-E6, Vero-SLAM, and H292 cells (mucoepidermoid carcinoma cells from human lungs) with patient blood and serum collected 3 days after symptom onset, but no virus isolate was obtained. As an alternative, 10 μL of the blood sample was also inoculated intracranially and intraperitoneally into 2-day-old suckling mice, which were then observed for 28 days for signs of illness. Neurologic signs developed 9–10 days after inoculation in 2 of the 20 mice; these 2 mice were euthanized 2 days later. To confirm the presence of the virus, we extracted total RNA from liver, spleen, and brains of the euthanized animals and used it as input in a specific RT-PCR designed to amplify a 2,188-bp fragment partially spanning the virus HN and L genes. Consistent with virus replication and observed neurologic signs, viral RNA was found in the brain but not in liver or spleen samples (Figure 3, panel A).
Brain homogenates from the euthanized mice were inoculated into fresh monolayers of Vero-E6 cells and H292 cells; 12 days after infection, a cytopathic effect, with cell rounding but no syncytia formation, became evident. Virus antigen was detected by immunofluorescence in both cell lines by using patient’s convalescent-phase serum, collected 50 days after symptom onset (Figure 3, panel B). Moreover, transmission electron microscopy used to examine virus morphology showed pleomorphic virions, consistent with those of paramyxoviruses (Figure 3, panel C).
Because the patient seemed to have acquired the infection during her African research expedition, where she had had extensive contact with rodents and bats, other persons who also come in contact with bats or rodents, such as field biologists, local residents, or ecotourists, might be at risk for infection. This potential public health threat prompted us to develop diagnostic assays for the rapid detection of Sosuga virus.
First, we developed a TaqMan real-time RT-PCR selective for the N gene and tested it on all available serum and blood samples from the patient. This test showed that the patient’s viremia peaked early in the course of the infection (cycle threshold 29.5 on day 3 after symptom onset), coinciding with the period of high fever and diverse irregularities in blood parameters (Table). By day 9, the viremia had decreased (cycle threshold 36.3); viremia was undetectable 11 days after symptom onset.
Second, we developed a new ELISA specific for Sosuga virus by using the virus recombinant nucleocapsid protein produced and purified from Escherichia coli. This assay was tested on all available serum samples from the patient (Table). Although IgG and IgM were not detectable on day 3 after symptom onset (titers <50), seroconversion (IgG and IgM titers >1,600) occurred 11 days after symptom onset. As expected, IgM levels later decreased (titer >400), and IgG levels remained high 50 days after symptom onset.
In addition, the new ELISA was tested for potential cross-reactivity with some common paramyxoviruses, including mumps and measles viruses. No cross-reactivity was detected on the ELISA plates when control serum from patients with high levels of IgG against mumps and measles viruses was used, a desired feature in a new diagnostic assay because most persons have IgG to these viruses as a result of vaccination or natural infection.
A severe disease affected a wildlife biologist shortly after her return from rural Africa to the United States. Because of the disease characteristics (high fever and blood abnormalities) and travel history, a viral hemorrhagic fever was suspected, and clinical samples were rushed to CDC for investigation of a possible high-risk virus. After molecular and serologic diagnostic assays ruled out several well-known human pathogens (e.g., filoviruses, arenaviruses, phleboviruses, flaviviruses, and rickettsiae) as the cause of the patient’s illness, a next-generation sequence approach was followed to detect a possible new infectious agent.
The combination of next-generation sequencing and metagenomic analysis identified a novel paramyxovirus; the virus genome was completely characterized by use of standard sequencing techniques. The complete virus sequence clearly indicated a relationship with other rubula-like viruses isolated from bats. Moreover, the novel virus was isolated from acute-phase serum samples by infecting suckling mice and propagating the virus in cell culture.
The specific molecular and serologic diagnostic assays that we developed will facilitate rapid identification of this novel infectious agent should new cases occur. We used these assays to retrospectively investigate all available clinical samples from the patient, and the results revealed periods of viremia and seroconversion.
Although the exact source of the patient’s infection remains unknown, the sequence similarity with bat-derived rubula-like viruses is highly suggestive. In recent years, a large number of diverse paramyxoviruses have been detected in bats (2,10), but only Nipah and Hendra viruses (genus Henipavirus) are known to cause severe disease in humans (11). An investigation to detect Sosuga virus in African bats is currently under way.
Dr Albariño is a senior research fellow at CDC in Atlanta, Georgia. His research is focused on different aspects of RNA viruses with the goal of developing new diagnostic techniques and evaluating potential vaccines.
Acknowledgment
We thank the Arbovirus Diagnostic Laboratory and Rickettsial Zoonoses Branch Reference Diagnostic Laboratory, CDC, for rule-out testing. We also thank Kathryn Roberts, Kim Dodd, Brock Martin, and JoAnn Coleman for their assistance with animal procedures and husbandry and Tatyana Klimova for her assistance during the editing process.
References
- Lamb RA, Parks GD. Paramixoviridae: the viruses and their replication. In: Fields BN, Knipe DM, Howley PM, editors. Fields virology. 5th ed. Philadelphia: Lippincott, Williams and Wilkins; 2007. p. 1449–96.
- Drexler JF, Corman VM, Muller MA, Maganga GD, Vallo P, Binger T, Bats host major mammalian paramyxoviruses. Nat Commun. 2012;3:796 .DOIPubMedGoogle Scholar
- Baker KS, Todd S, Marsh GA, Crameri G, Barr J, Kamins AO, Novel, potentially zoonotic paramyxoviruses from the African straw-colored fruit bat Eidolon helvum. J Virol. 2013;87:1348–58. DOIPubMedGoogle Scholar
- Lau SK, Woo PC, Wong BH, Wong AY, Tsoi HW, Wang M, Identification and complete genome analysis of three novel paramyxoviruses, Tuhoko virus 1, 2 and 3, in fruit bats from China. Virology. 2010;404:106–16. DOIPubMedGoogle Scholar
- McMullan LK, Folk SM, Kelly AJ, MacNeil A, Goldsmith CS, Metcalfe MG, A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834–41. DOIPubMedGoogle Scholar
- McMullan LK, Frace M, Sammons SA, Shoemaker T, Balinandi S, Wamala JF, Using next generation sequencing to identify yellow fever virus in Uganda. Virology. 2012;422:1–5. DOIPubMedGoogle Scholar
- Palacios G, Quan PL, Jabado OJ, Conlan S, Hirschberg DL, Liu Y, Panmicrobial oligonucleotide array for diagnosis of infectious diseases. Emerg Infect Dis. 2007;13:73–81. DOIPubMedGoogle Scholar
- Huson DH, Auch AF, Qi J, Schuster SC. MEGAN analysis of metagenomic data. Genome Res. 2007;17:377–86. DOIPubMedGoogle Scholar
- Bergeron É, Chakrabarti AK, Bird BH, Dodd KA, McMullan LK, Spiropoulou CF, Reverse genetics recovery of Lujo virus and role of virus RNA secondary structures in efficient virus growth. J Virol. 2012;86:10759–65. DOIPubMedGoogle Scholar
- Baker KS, Todd S, Marsh G, Fernandez-Loras A, Suu-Ire R, Wood JL, Co-circulation of diverse paramyxoviruses in an urban African fruit bat population. J Gen Virol. 2012;93:850–6. DOIPubMedGoogle Scholar
- Eaton BT, Mackenzie JS, Wang L. Henipaviruses. In: Fields BN, Knipe DM, Howley PM, editors. Fields virology. 5th ed. Philadelphia: Lippincott, Williams and Wilkins; 2007. p. 1587–600.
Figures
Table
Cite This ArticleTable of Contents – Volume 20, Number 2—February 2014
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Ute Ströher, Centers for Disease Control and Prevention, 1600 Clifton Rd NE, Mailstop A26, Atlanta, GA 30333, USA
Top