Volume 20, Number 5—May 2014
Research
Molecular Characterization of Cryptically Circulating Rabies Virus from Ferret Badgers, Taiwan
Abstract
After the last reported cases of rabies in a human in 1959 and a nonhuman animal in 1961, Taiwan was considered free from rabies. However, during 2012–2013, an outbreak occurred among ferret badgers in Taiwan. To examine the origin of this virus strain, we sequenced 3 complete genomes and acquired multiple rabies virus (RABV) nucleoprotein and glycoprotein sequences. Phylogeographic analyses demonstrated that the RABV affecting the Taiwan ferret badgers (RABV-TWFB) is a distinct lineage within the group of lineages from Asia and that it has been differentiated from its closest lineages, China I (including isolates from Chinese ferret badgers) and the Philippines, 158–210 years ago. The most recent common ancestor of RABV-TWFB originated 91–113 years ago. Our findings indicate that RABV could be cryptically circulating in the environment. An understanding of the underlying mechanism might shed light on the complex interaction between RABV and its host.
Rabies is possibly one of the oldest zoonotic diseases. It is caused by the rabies virus (RABV), a neurotropic virus in the family Rhabdoviridae, genus Lyssavirus. Except for a small number of countries and regions, particularly islands, RABV is found worldwide. The virus infects nearly all warm-blooded animals and causes severe neurologic signs, which almost invariably lead to death (1). It was estimated that worldwide in 2010, the disease caused >60,000 human deaths, primarily in Africa and Asia (2). Although dogs are considered the principal host of RABV in developing countries, the virus is also dispersed among many species of wild carnivora and chiroptera, especially in those countries of Europe and North America that have well-established vaccination programs (3). Mustelids, including various species of the genera Melogale, Meles, and Mellivora of the weasel family Mustelidae, can carry RABV (4–6). In southeastern China, Chinese ferret badgers (CNFB; Melogale moschata moschata) have been associated with human rabies for many years and are considered to be a primary host in this region (7–9).
After what were considered to be the last reported cases of rabies in a human and a nonhuman animal in 1959 and 1961, respectively, Taiwan was rabies free for >50 years until the 2012–2013 outbreak of ferret badger–associated rabies. During May 2012–January 2013, through a government-supported program of routine disease surveillance of free-range dead wild animals that had been killed by vehicles or were receiving treatment for injuries and/or illness at the wildlife first aid station, 3 dead Taiwan ferret badgers (TWFB; M. moschata subaurantiaca) were submitted to the School of Veterinary Medicine, National Taiwan University, for further examination.
Pathologic examination revealed nonsuppurative meningoencephalomyelitis with formation of eosinophilic intracytoplasmic inclusion bodies in all 3 animals; reverse transcription PCR and immunohistochemical staining excluded the possibility of infection with the canine distemper virus. However, the results of fluorescence antibody testing, immunohistochemical staining, and reverse transcription PCR, followed by sequencing for RABV, were positive (H.-Y. Chiou, unpub. data). After the rabies diagnoses for the initial 3 ferret badgers were confirmed, by the end of August of 2013, rabies had been diagnosed by fluorescence antibody testing for an additional 105 dead or ill and euthanized ferret badgers and 1 shrew.
Our objective in this study was to clarify whether the current outbreak of the TWFB–associated rabies is an emerging, a reemerging, or a cryptically circulating disease. We investigated the possible origin of this outbreak and its relations with CNFB-associated rabies in mainland China via genomic organization and characterization and analysis of genetic diversity and phylogeographic origin of RABV-TWFB. In addition, we propose a mechanism that might be contributing to the limited host range of RABV-TWFB.
Animals and Specimen Collection
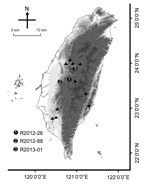
During May 2012–January 2013, three ill TWFB were collected from different regions of central Taiwan (Figure 1). One was in the Xitou nature education area at Lugu Township, Nantou County (R2012–26); one was in Gukeng Township, Yunlin County (R2012–88); and one was in Yuchih Township, Nantou County (R2013–01). These 3 TWFB, respectively, showed the following clinical signs: emaciation, coma, paddling, loss of pain response, reduced body temperature, and a 2-cm skin wound on the chin; extreme weakness and inability to move; and signs of weakness and respiratory signs, including labored breathing and increased breath sounds with hypersalivation and exudation of foamy fluid from the mouth and nose. Initial supportive treatment was provided at the wildlife first aid station, but the ferret badgers died within 1–3 days, and their carcasses were submitted to the School of Veterinary Medicine, National Taiwan University, for routine disease surveillance. Full necropsy was performed, during which half of the left cerebral hemisphere was collected from each animal and stored at −80°C for subsequent nucleic acid extraction. Representative tissue samples were taken from all major organs and fixed in 10% neutral buffered formalin for histopathologic examination.
Sample Preparation and Genome Sequencing
Approximately 25 mg of brain specimen from each animal was homogenized, and 1 mL of TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was added. Total RNA was extracted by using an RNeasy Mini Kit (QIAGEN, Valencia, CA, USA), and cDNA was synthesized by using a Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostics, Indianapolis, IN, USA) according to the manufacturer’s instructions. To amplify the whole genome, we used 19 pairs of primers (Table 1), including the forward primer for the 5′ end and the reverse primer for the 3′ end designed to be complementary to the respective ends of the genome, as described (10).
Sequence Analyses and Phylogenetic Reconstruction
Sequences were assembled by using the Seqman program (Lasergene 8, Madison, WI, USA) (GenBank accession nos. KF620487–KF620489) and then aligned by using the ClustalW program (11). The genetic distance was estimated by using the Kimura 2-parameter substitution model implemented in MEGA version 5.0 (12). The nucleotide diversity within populations was calculated by using DnaSP version 5.0 (13). To test for the deviation of neutral expectation, we conducted the Tajima D (14) and the Fu and Li D* (15) tests implemented in DnaSP. Significance was assessed by 104 coalescent simulations (13).
To investigate the phylogenetic position of RABV-TWFB isolates, we included 24 complete RABV genomes representing the 3 major phylogenetic groups (16). For global phylogeny of RABV, we analyzed 218 full-length (1,335-nt) sequences of the nucleoprotein (N) gene, including 11 sequences from Taiwan. We also analyzed 125 full-length (1,575-nt) sequences of the glycoprotein (G) gene, including 13 sequences from Taiwan (17). For each gene, phylogenetic trees were inferred by using maximum-likelihood and Bayesian inference methods.
The maximum-likelihood analysis was conducted by using PhyML 3.0 online (18); the starting tree was derived from the neighbor-joining method, and the nearest neighbor interchange topology search option was used. The nucleotide substitution model for phylogenetic reconstruction was determined by using the Akaike information criterion implemented in jModeltest 0.1.1 (19). The method of Bayesian inference was performed by using MrBayes version 3.1.2 (20). Analyses were initiated with random starting trees, and Metropolis-coupled Markov chain Monte Carlo (MCMC) analyses were run for 1 × 106 generations and sampled every 100 generations. The steady state of the log-likelihood was reached at ≈20,000 generations. Subsequently, the first 201 trees were excluded and the remaining 9,800 trees were retained to compute a 50% majority-rule consensus tree.
Divergence Dating
The divergence time between different viral lineages and the time to the most recent common ancestor (TMRCA) of virus isolates were estimated by using an established Bayesian MCMC approach implemented in BEAST version 1.7 (21). The analysis was performed by using the general time-reversible model of nucleotide substitution assuming an uncorrelated lognormal molecular clock (22). We linked substitution rates for the first and second codon positions and allowed independent rates in the third codon position. The molecular clocks were 2.3 × 10–4 (range 1.1–3.6 × 10–4) and 3.9 × 10–4 (1.2–6.5 × 10–4) substitutions/site/year for N and G genes, respectively (17). A slightly faster clock, 4.3 × 10–4 (3.1–5.6 × 10–4) substitutions/site/year for N gene (23), was also used in a separate analysis.
Because a previous study revealed that the population dynamics of RABV supported a model of constant population size through time (17), we restricted our analysis to this demographic model. For each analysis, we performed 2 independent runs with 2 × 107 MCMC steps, of which the first 10% were discarded as burn-in. To confirm that both were sampling the same distribution, we compared and then combined the results. Log files were checked by using Tracer (http://beast.bio.ed.ac.uk/Tracer), and the effective sample size for each parameter was >300, which is adequate according to the authors of BEAST software.
Genomic Organization and Characterization of RABV-TWFB
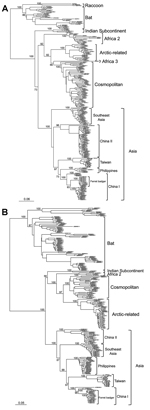
Similar to previous analyses (16,17), our phylogenetic analysis that used whole RABV genomes revealed 3 major groups with high bootstrap support (Figure 2). Although the 3 RABV-TWFB isolates are clustered within the Asia group, composed of 3 distinct lineages, (China I [including CNFB], China II [16], and Southeast Asia), they do not appear close to any of the 3 lineages. More noteworthy, the 3 isolates of RABV-TWFB are not close to those of RABV-CNFB, indicating that they may have originated independently.
The genome of RABV-TWFB is 11,923 nt long and encodes 5 proteins. The nucleotide lengths of different genomic regions are within the range of variations in different Asia lineages (Table 2), except the matrix protein (M)-G intergenic region, which is 1 nt longer than in the rest of the lineages from Asia (212 vs. 211). Within the group of Asia lineages, the most conserved protein is M, followed by N, the virion-associated RNA polymerase (L), and G; the least conserved is phosphoprotein (P) (Table 3). Among the RABV groups, however, N becomes the most conserved followed by L, M, G, and P. The RABV-TWFB is closest to China I lineage in the N, P, and L gene regions, but it is closest to RABV-CNFB in the M and G gene regions.
The genetic variations across the whole genome among different lineages can be viewed in a sliding window analysis (Figure 3). Within N, there seems to be a conserved central domain previously identified in RABV at residues 182–328 (24), which is also conserved in RABV-TWFB. The P, the last quarter of G and G–L intergenic regions, and the last part of L are more variable among different lineages of RABV than is the rest of the genome. The conserved M is functional in viral assembly and budding (25); is involved in the regulation of transcription and replication of viral RNA (26); and has been reported to induce apoptosis (27), suggesting its role in host-cell interplay. The involvement of M in multiple interactions explains its conservation among lineages. G is responsible for cell attachment and fusion and is the main viral protein responsible for the induction of neutralization antibodies and cell-mediated immune responses. The region between aa 189 and aa 214, proposed to be needed for G binding to the nicotinic acetylcholine receptor (28), is relatively conserved in the dataset. Nevertheless, 3 substitutions (N194Y, R196K, and G203E) are found exclusively in RABV-TWFB G. In L, Poch et al. (29) recognized 6 conserved blocks, including B1 (233–424), B2 (504–608), B3 (609–832), B4 (890–1061), B5 (1091–1326), and B6 (1674–1749) (29). In addition, 2 regions, L1 (1418–1515) and L2 (1884–1961), are also conserved across lyssaviruses (30). In RABV-TWFB, the B4 and L1 regions in L are variable, and the rest of the blocks are conserved (Figure 3).
Genetic Diversity and Phylogeographic Origin of RABV-TWFB
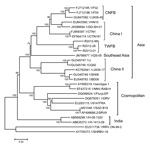
The data shown in Figure 2 indicate that RABV-TWFB is a distinct lineage within the Asia group of viruses. To further explore the detailed origin of RABV-TWFB, we included the representative N and G sequences of RABV from human and various animal species for analysis (17,31). Because maximum-likelihood and Bayesian inference methods yielded similar topologies, we report only the results derived from the former. Both N and G gene trees support the conclusion that RABV-TWFB is a distinct lineage within the Asia group, clustered with the China I lineage, including RABV-CNFB, and sequences from the Philippines (Figure 4, Appendix).
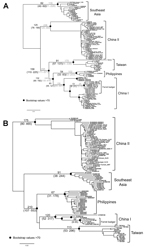
Divergence time was estimated by using a Bayesian coalescent approach. In this analysis, we included only sequences of the Asia group. On the basis of the molecular clock of 4.3 × 10–4/site/year for N gene (23), the substitution rate at the third codon position is 1.1 × 10–3/site/year, and RABV-TWFB was separated from China I and the Philippines isolates 158 years ago with 95% highest posterior density (HPD) ranging from 110 to 225 years (Figure 5, panel A, Appendix). The divergence between China I and the Philippines isolates occurred 132 (95% HPD, 90–192) years ago, which is similar to previous estimations (23,32). The TMRCA of isolates from Taiwan was 91 years (95% HPD, 57–137). A similar timescale, with overlapping 95% HPD, was derived by using the molecular clock of 2.3 × 10–4/site/year for N gene (17) (Figure 5, panel A, hatched numbers, Appendix). The mean substitution rate for G gene sequences was 3.5 × 10–4/site/year (7.8 × 10–4 for the third codon position), and the divergence of RABV-TWFB, China I, and the Philippines isolates was initiated 210 (107–553) years ago, and the TMRCA of isolates from Taiwan was 113 (53–296) years (Figure 5, panel B, Appendix). It is notable that the TMRCA of RABV-TWFB was more ancient than that of several distinct lineages in Figure 5, Appendix. For example, the TMRCA was 62–116 years for the Southeast Asia lineage and 54–102 years for RABV of the Philippines. The origin of RABV-CNFB was relatively recent; TMRCA was 13–25 years.
The nucleotide diversities of RABV-TWFB are 3.14% for the N and 4.21% for the G genes (Table 4), which are almost 5 times higher than those of RABV-CNFB, which are 0.67% for the N and 0.87% for G genes. For comparison purposes, the 65 N and 232 G gene sequences of RABV isolates from the Philippines were also included for analysis (33). The nucleotide diversities are 2.00% for the N gene and 2.57% for the G gene. The results of both the Tajima D and the Fu and Li D* tests are not significant for RABV-TWFB, indicating that the viral population is under neutral equilibrium, which in turn suggests that RABV was not recently introduced to TWFB. In contrast, the results of the Tajima D and the Fu and Li D* tests are significantly negative for the sequences of RABV-CNFB and sequences from the Philippines isolates, which are caused by an excess of low-frequency mutation or by differentiation among populations.
The Ancient Origin of RABV-TWFB
We sequenced and characterized a RABV strain, RABV-TWFB, recently isolated from ferret badgers in Taiwan. Our data showed that RABV-TWFB is clustered with sequences from the Philippines, China I, and RABV-CNFB. This relationship is strongly supported on the basis of multiple sequences of the N and G genes and of the complete genome (Figures 2 and 4, Appendix). Of ferret badger isolates, RABV-TWFB and RABV-CNFB come from phylogenetically distinct lineages, indicating that multiple RABV colonization events in this species probably occurred. A major question addressed in this study is whether RABV was recently introduced into the population of TWFB or perpetuated in TWFB without revealing its presence pathogenically after it was first introduced in the ancient past. Our divergence dating showed that the RABV has been circulating in TWFB for ≈100 years.
Our divergence and TMRCA estimations have a few potential sources of error. First, the RABV isolates from Taiwan might have originated from several introduction events, including the probability that multiple viral lineages occurred in the recent past and that the inflated TMRCA resulted from the combination of different, highly differentiated virus strains. Nevertheless, all isolates from Taiwan formed a monophyletic lineage distinct from other virus isolates. Unless several undetected virus strains were circulating around Taiwan, which is highly unlikely, the phylogenetic analyses support the existence of only 1 origin of RABV-TWFB.
Second, the ancient estimates could have resulted from the application of an inadequate molecular clock. However, the nucleotide substitution (mutation) rates of 2.3–4.3 × 10–4 and 3.9 × 10–4/site/year, for the N and G genes, respectively, used in the study reported here are in agreement with findings of other studies of lyssavirus evolution (17,23,32,34,35). In a study of RABV in bats, Streicker et al. (34) found that the nucleotide substitution rates in the third codon position, which are predominately silent (synonymous) substitutions, among viral lineages in different bat species spanned 8.3 × 10–5-2.1 × 10–3/site/year. Our estimations of mutations of 1.1 × 10–3 and 7.8 × 10–4/sites/year for the third codon position of the N and G genes, respectively, are actually close to the upper boundary of their estimations. Therefore, our results should be conservative.
Third, RABV-TWFB exhibits high nucleotide diversity in the N and G genes. Notably, 232 G gene sequences collected from a large area of the Philippines showed nucleotide diversity that was two thirds that of RABV-TWFB. Taken together, all current genetic evidence supports the hypothesis of the ancient origin of RABV-TWFB. In addition, RABV-TWFB has been maintained in a large population for a long time.
Last, our recent retrospective study that used the archived formalin-fixed and paraffin-embedded brain tissues of ferret badgers, kindly provided by various institutes, demonstrated that the current earliest TWFB-associated RABV infection could be traced back to 2004 (H.-Y. Chiou, unpub. data), representing the oldest specimens that we have so far. That finding is consistent with the notion of a long history of RABV-TWFB in Taiwan.
Mutations in the G Gene of RABV-TWFB
The ancient history of RABV-TWFB raises 2 issues. First, because for the past 50 years Taiwan was believed to have been free from rabies, learning that the virus must have been cryptically circulating in the environment for such a long time is surprising. Because previous rabies surveillance was mainly focused on dogs and bats (www.baphiq.gov.tw), cases in remote areas might have gone unnoticed. However, Taiwan is an island with a high population density; 23 million persons live in an area of 36,188 km2, and for rabies cases to have gone unnoticed for >50 years would be very unusual. Second, according to a recent survey about wildlife, the ferret badger population has been increasing in the past 5 years (L.-K. Lin, pers. comm.). Therefore, despite the ancient history of the ferret badger’s association with RABV, the fact that its population is seemingly unaffected by infection with RABV is perplexing.
Except for 1 isolate from a shrew, all RABV isolates in the recent rabies outbreak in Taiwan have come from ferret badgers. The close relationship between the shrew RABV and ferret badger RABV collected from the same area suggests that the former probably resulted from spillover from the latter (Figure 5, panel B, Appendix). According to the most recent rabies surveillance data, the ferret badger is probably the only source of RABV in the current outbreak in Taiwan. Speculation that this RABV strain has adapted to and has been circulating in TWFB for a long time is reasonable. Its ability to transmit across species (e.g., ferret badger to shrew) is, thus, worthy of further investigation.
Among the multiple substitutions in RABV-TWFB genome that distinguish it from other virus strains, several substitutions in G (i.e., N194Y, R196K, and G203E) might merit additional attention. It has been demonstrated that a single amino acid mutation, N194K, in the nonpathogenic RABV vaccine strain SAD B19 was solely responsible for its increased pathogenicity. The increased pathogenicity is caused by increased virus spread in vivo and faster internalization of the virus into cells (36), both of which are consistent with the notion that G plays a major role in RABV pathogenesis. When the amino acid N was exchanged with amino acid S at position 194 (N194S), the pathogenic phenotype was reversed (36,37). Faster internalization of the virus into cells after N194K substitution might suggest that this region has some role in host cell binding. Evidence is strong that the muscular form of the nicotinic acetylcholine receptor, the neuronal cell adhesion molecule, and the p75 neurotrophin receptor serve as receptor sites for RABV binding and/or facilitate its entry into host cells (38,39). The 3 above-mentioned substitutions in RABV-TWFB are located in the region (aa 189 and 214) proposed to be needed for the binding of the G to the nicotinic acetylcholine receptor (28).
In our analysis of 113 G gene sequences from dog-associated RABV, no amino acid substitution was observed at the above-mentioned sites. In a total of 120 G gene sequences from bat-associated RABV (40), 8 N194T (6.7%), 2 N194S (1.7%), and 36 R196K (30.0%) amino acid substitutions were revealed with no amino acid change at G203. Taken together, the 3 aa substitutions (N194Y, R196K, and G203E) found in all 13 G gene sequences of RABV-TWFB are unique and worthy of further investigation.
Dr Chiou is a chief pathology resident and a PhD candidate in the School of Veterinary Medicine, National Taiwan University. Her primary research interests include disease surveillance and monitoring of wildlife and zoonotic diseases.
Acknowledgments
We thank the Animal Health Research Institute, Council of Agriculture, Executive Yuan (ROC) for providing 8 and 10 full-length sequences of the N and G genes, respectively, for phylogenetic analysis. Special thanks go also to the Taiwan Endemic Species Research Institute, Council of Agriculture, for collection and submission of carcasses of free-range wildlife.
This research was supported in part by grants 101AS-10.1.2-BQ-B1(1) and 102AS-10.1.1-BQ-B1(1) from the Bureau of Animal and Plant Health Inspection and Quarantine, Council of Agriculture, Executive Yuan, Taiwan (ROC) (to V.F.P.) and NSC102-2313-B-002-062 from the Ministry of Science and Technology, Taiwan (ROC) (to H.-Y.W.).
References
- Jackson AC. Pathogenesis. In: Jackson AC, Wunner WH, editors. Rabies. London: Elsevier Academic Press; 2007.
- World Health Organization. WHO Expert Consultation on Rabies: second report. Geneva. Organization. 2013;•••:1–139.
- Wandeler A. Virus infections of non-domestic carnivores: rabies virus. In: Appel MJ, editor. Virus infections of carnivores. Amsterdam: Elsevier Science Publishers; 1987. p. 449–61.
- Barnard BJH. The role played by wildlife in the epizootiology of rabies in South Africa and South-West Africa. Onderstepoort J Vet Res. 1979;46:155–63 .PubMedGoogle Scholar
- Smith GC, Wilkinson D. Modelling disease spread in a novel host: rabies in the European badger Meles meles. J Appl Ecol. 2002;39:865–74. DOIGoogle Scholar
- Wandeler A, Wachendorfer G, Forster U, Krekel H, Muller J, Steck F. Rabies in wild carnivores in central Europe. II. Virological and serological examinations. Zentralbl Veterinarmed B. 1974;21:757–64 . DOIPubMedGoogle Scholar
- Liu Y, Zhang SF, Wu XF, Zhao JH, Hou YL, Zhang F, Ferret badger rabies origin and its revisited importance as potential source of rabies transmission in southeast China. BMC Infect Dis. 2010;10:234. DOIPubMedGoogle Scholar
- Zhenyu G, Zhen W, Enfu C, Fan H, Junfen L, Yixin L, Human rabies cluster following badger bites, People’s Republic of China. Emerg Infect Dis. 2007;13:1956–7. DOIPubMedGoogle Scholar
- Zhang S, Tang Q, Wu XF, Liu Y, Zhang F, Rupprecht CE, Rabies in ferret badgers, southeastern China. Emerg Infect Dis. 2009;15:946–9 . DOIPubMedGoogle Scholar
- Lei YL, Wang XG, Liu FM, Chen XY, Ye BF, Mei JH, Complete genome sequencing and analyses of rabies viruses isolated from wild animals (Chinese ferret-badger) in Zhejiang Province [in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi. 2009;30:824–8 .PubMedGoogle Scholar
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–80. DOIPubMedGoogle Scholar
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9. DOIPubMedGoogle Scholar
- Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–2. DOIPubMedGoogle Scholar
- Tajima F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 1989;123:585–95 .PubMedGoogle Scholar
- Fu YX, Li WH. Statistical tests of neutrality of mutations. Genetics. 1993;133:693–709 .PubMedGoogle Scholar
- He CQ, Meng SL, Yan HY, Ding NZ, He HB, Yan JX, Isolation and identification of a novel rabies virus lineage in China with natural recombinant nucleoprotein gene. PLoS ONE. 2012;7:e49992. DOIPubMedGoogle Scholar
- Bourhy H, Reynes JM, Dunham EJ, Dacheux L, Larrous F, Huong VT, The origin and phylogeography of dog rabies virus. J Gen Virol. 2008;89:2673–81. DOIPubMedGoogle Scholar
- Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. DOIPubMedGoogle Scholar
- Posada D. jModelTest: phylogenetic model averaging. Mol Biol Evol. 2008;25:1253–6. DOIPubMedGoogle Scholar
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–4. DOIPubMedGoogle Scholar
- Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol. 2012;29:1969–73. DOIPubMedGoogle Scholar
- Drummond AJ, Ho SY, Phillips MJ, Rambaut A. Relaxed phylogenetics and dating with confidence. PLoS Biol. 2006;4:e88. DOIPubMedGoogle Scholar
- Meng S, Sun Y, Wu X, Tang J, Xu G, Lei Y, Evolutionary dynamics of rabies viruses highlights the importance of China rabies transmission in Asia. Virology. 2011;410:403–9. DOIPubMedGoogle Scholar
- Kissi B, Tordo N, Bourhy H. Genetic polymorphism in the rabies virus nucleoprotein gene. Virology. 1995;209:526–37. DOIPubMedGoogle Scholar
- Mebatsion T, Weiland F, Conzelmann KK. Matrix protein of rabies virus is responsible for the assembly and budding of bullet-shaped particles and interacts with the transmembrane spike glycoprotein G. J Virol. 1999;73:242–50 .PubMedGoogle Scholar
- Finke S, Mueller-Waldeck R, Conzelmann KK. Rabies virus matrix protein regulates the balance of virus transcription and replication. J Gen Virol. 2003;84:1613–21. DOIPubMedGoogle Scholar
- Kassis R, Larrous F, Estaquier J, Bourhy H. Lyssavirus matrix protein induces apoptosis by a TRAIL-dependent mechanism involving caspase-8 activation. J Virol. 2004;78:6543–55. DOIPubMedGoogle Scholar
- Lentz TL, Wilson PT, Hawrot E, Speicher DW. Amino acid sequence similarity between rabies virus glycoprotein and snake venom curaremimetic neurotoxins. Science. 1984;226:847–8. DOIPubMedGoogle Scholar
- Poch O, Blumberg BM, Bougueleret L, Tordo N. Sequence comparison of five polymerases (L proteins) of unsegmented negative-strand RNA viruses: theoretical assignment of functional domains. J Gen Virol. 1990;71:1153–62. DOIPubMedGoogle Scholar
- Kuzmin IV, Wu X, Tordo N, Rupprecht CE. Complete genomes of Aravan, Khujand, Irkut and West Caucasian bat viruses, with special attention to the polymerase gene and non-coding regions. Virus Res. 2008;136:81–90. DOIPubMedGoogle Scholar
- Zhang S, Zhao J, Liu Y, Fooks AR, Zhang F, Hu R. Characterization of a rabies virus isolate from a ferret badger (Melogale moschata) with unique molecular differences in glycoprotein antigenic site III. Virus Res. 2010;149:143–51. DOIPubMedGoogle Scholar
- Gong W, Jiang Y, Za Y, Zeng Z, Shao M, Fan J, Temporal and spatial dynamics of rabies viruses in China and Southeast Asia. Virus Res. 2010;150:111–8. DOIPubMedGoogle Scholar
- Saito M, Oshitani H, Orbina JR, Tohma K, de Guzman AS, Kamigaki T, Genetic diversity and geographic distribution of genetically distinct rabies viruses in the Philippines. PLoS Negl Trop Dis. 2013;7:e2144. DOIPubMedGoogle Scholar
- Streicker DG, Lemey P, Velasco-Villa A, Rupprecht CE. Rates of viral evolution are linked to host geography in bat rabies. PLoS Pathog. 2012;8:e1002720. DOIPubMedGoogle Scholar
- Biek R, Henderson JC, Waller LA, Rupprecht CE, Real LA. A high-resolution genetic signature of demographic and spatial expansion in epizootic rabies virus. Proc Natl Acad Sci U S A. 2007;104:7993–8. DOIPubMedGoogle Scholar
- Faber M, Faber ML, Papaneri A, Bette M, Weihe E, Dietzschold B, A single amino acid change in rabies virus glycoprotein increases virus spread and enhances virus pathogenicity. J Virol. 2005;79:14141–8. DOIPubMedGoogle Scholar
- Faber M, Li J, Kean RB, Hooper DC, Alugupalli KR, Dietzschold B. Effective preexposure and postexposure prophylaxis of rabies with a highly attenuated recombinant rabies virus. Proc Natl Acad Sci U S A. 2009;106:11300–5. DOIPubMedGoogle Scholar
- Tuffereau C, Schmidt K, Langevin C, Lafay F, Dechant G, Koltzenburg M. The rabies virus glycoprotein receptor p75NTR is not essential for rabies virus infection. J Virol. 2007;81:13622–30. DOIPubMedGoogle Scholar
- Kuzmin IV, Shi M, Orciari LA, Yager PA, Velasco-Villa A, Kuzmina NA, Molecular inferences suggest multiple host shifts of rabies viruses from bats to mesocarnivores in Arizona during 2001–2009. PLoS Pathog. 2012;8:e1002786 . DOIPubMedGoogle Scholar
Figures
Tables
Cite This Article1Joint senior authors who contributed equally to this article.
Table of Contents – Volume 20, Number 5—May 2014
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Address for correspondence: Victor Fei Pang, Graduate Institute of Molecular and Comparative Pathobiology, School of Veterinary Medicine, National Taiwan University, No. 1, Sec. 4, Roosevelt Rd., Taipei 10617, Taiwan, ROCAddress for correspondence: Victor Fei Pang, Graduate Institute of Molecular and Comparative Pathobiology, School of Veterinary Medicine, National Taiwan University, No. 1, Sec. 4, Roosevelt Rd., Taipei 10617, Taiwan, ROCAddress for correspondence: Victor Fei Pang, Graduate Institute of Molecular and Comparative Pathobiology, School of Veterinary Medicine, National Taiwan University, No. 1, Sec. 4, Roosevelt Rd., Taipei 10617, Taiwan, ROC
Top