Volume 20, Number 9—September 2014
Letter
Invasive Infection Caused by Carbapenem-Resistant Acinetobacter soli, Japan
Figure
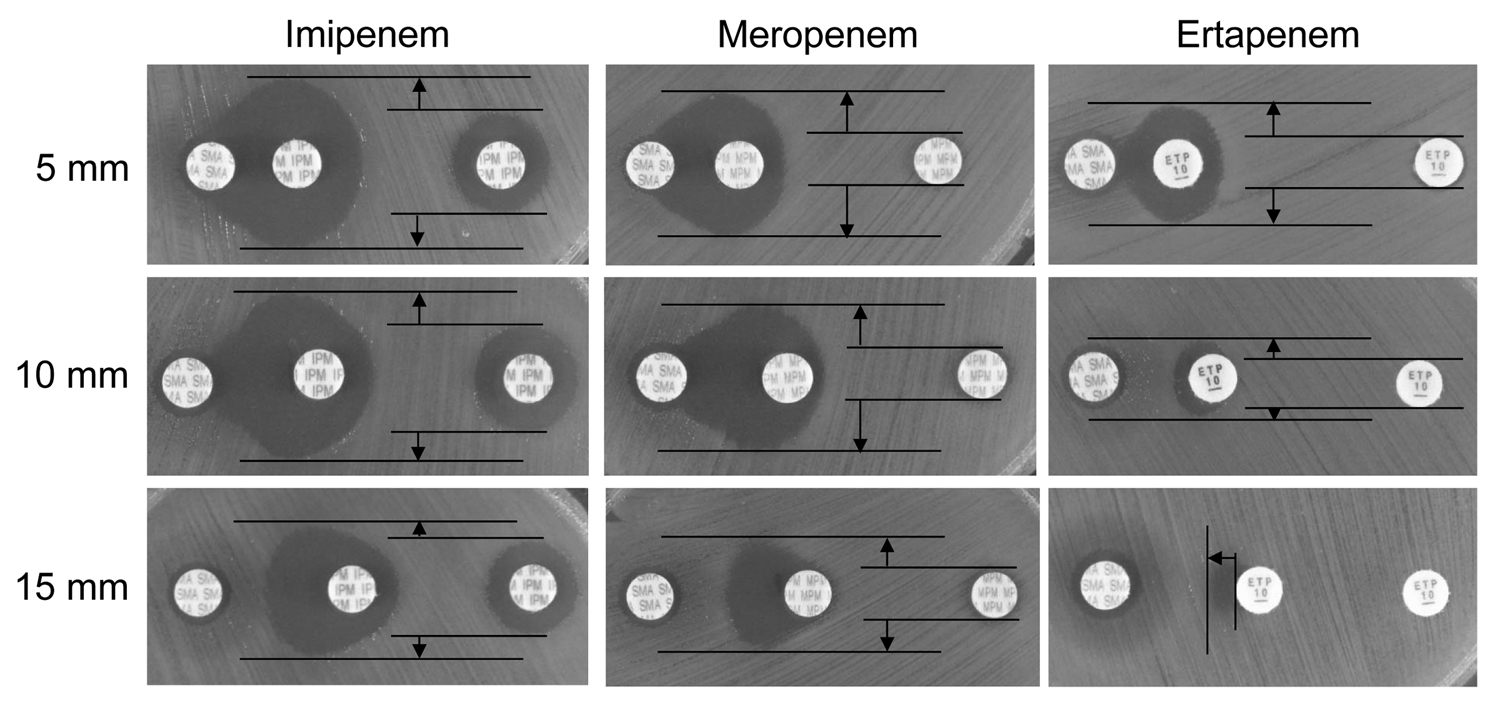
Figure. Results of double-disk synergy testing of the Acinetobacter soli isolate HK001 identified in a man in Japan. Testing was performed by using disks containing sodium mercaptoacetic acid (SMA) and the carbapenems imipenem, meropenem, and ertapenem. Apparent expansion of growth inhibition zone around a carbapenem disk placed near a SMA disk compared with that around a disk of carbapenem alone is seen on Mueller-Hinton agar if the isolate produces metallo-β-lactamases (2,3). When the edge-to-edge distance between 2 disks containing a carbapenem and SMA, respectively, was kept at 5 mm, expansion of the growth inhibition zone became clearer than for those kept at a distance of 10 mm and 15 mm, regardless of carbapenems used. Vertical expansion of growth inhibition zones by the effect of SMA is indicated by arrows; ertapenem gave the clearest result when the disk distance was kept at 5 mm (top right panel), even though A. soli HK001 co-produces oxacillinase 58–like carbapenemase, which is hardly inhibited by SMA.
References
- Yamamoto M, Nagao M, Matsumura Y, Hotta G, Matsushima A, Ito Y, Regional dissemination of Acinetobacter species harboring metallo-β-lactamase genes in Japan. Clin Microbiol Infect. 2013;19:729–36 . DOIPubMedGoogle Scholar
- Arakawa Y, Shibata N, Shibayama K, Kurokawa H, Yagi T, Fujiwara H, Convenient test for screening metallo-β-lactamase-producing gram-negative bacteria by using thiol compounds. J Clin Microbiol. 2000;38:40–3 .PubMedGoogle Scholar
- Hattori T, Kawamura K, Arakawa Y. Comparison of test methods for detecting metallo-β-lactamase-producing Gram-negative bacteria. Jpn J Infect Dis. 2013;66:512–8 .PubMedGoogle Scholar
- Malhotra J, Anand S, Jindal S, Rajagopal R, Lal R. Acinetobacter indicus sp. nov., isolated from a hexachlorocyclohexane dump site. Int J Syst Evol Microbiol. 2012;62:2883–90. DOIPubMedGoogle Scholar
- Kim D, Baik KS, Kim MS, Park SC, Kim SS, Rhee MS, Acinetobacter soli sp. nov., isolated from forest soil. J Microbiol. 2008;46:396–401. DOIPubMedGoogle Scholar
- Pellegrino FL, Vieira VV, Baio PV, dos Santos RM, dos Santos AL, Santos NG, Acinetobacter soli as a cause of bloodstream infection in a neonatal intensive care unit. J Clin Microbiol. 2011;49:2283–5. DOIPubMedGoogle Scholar
- Endo S, Yano H, Kanamori H, Inomata S, Aoyagi T, Hatta M, High frequency of Acinetobacter soli among Acinetobacter isolates causing bacteremia at a Japanese tertiary hospital. J Clin Microbiol. 2014;52:911–5. DOIPubMedGoogle Scholar
- El Salabi A, Borra PS, Toleman MA, Samuelsen Ø, Walsh TR. Genetic and biochemical characterization of a novel metallo-β-lactamase, TMB-1, from an Achromobacter xylosoxidans strain isolated in Tripoli, Libya. Antimicrob Agents Chemother. 2012;56:2241–5. DOIPubMedGoogle Scholar
- Suzuki S, Matsui M, Suzuki M, Sugita A, Kosuge Y, Kodama N, Detection of Tripoli metallo-β-lactamase 2 (TMB-2), a variant of blaTMB-1, in clinical isolates of Acinetobacter spp. in Japan. J Antimicrob Chemother. 2013;68:1441–2. DOIPubMedGoogle Scholar
- Kumsa B, Socolovschi C, Parola P, Rolain JM, Raoult D. Molecular detection of Acinetobacter species in lice and keds of domestic animals in Oromia Regional State, Ethiopia. PLoS ONE. 2012;7:e52377. DOIPubMedGoogle Scholar