Volume 21, Number 1—January 2015
Dispatch
Continuing Effectiveness of Serogroup A Meningococcal Conjugate Vaccine, Chad, 2013
Figure 2
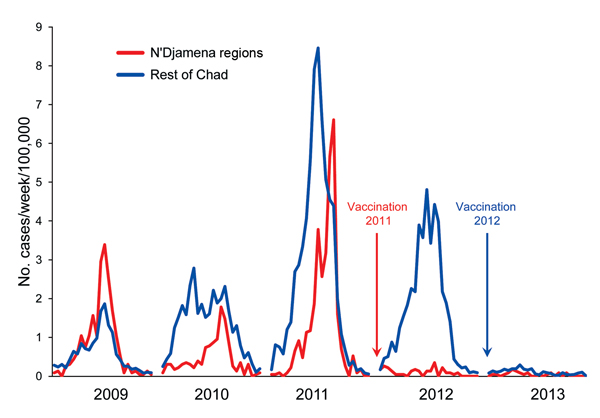
Figure 2. Incidence (no. cases/100,000 population) during weeks 1–26 of reported cases of meningitis in regions of Chad where persons 1–29 years of age were vaccinated with serogroup A meningococcal polysaccharide/tetanus toxoid conjugate vaccine at the end of 2011 and in 2012.
Page created: December 19, 2014
Page updated: December 19, 2014
Page reviewed: December 19, 2014
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.