Volume 21, Number 1—January 2015
Dispatch
Oseltamivir-Resistant Influenza A(H1N1)pdm09 Viruses, United States, 2013–14
Cite This Article
Citation for Media
Abstract
We report characteristics of oseltamivir-resistant influenza A(H1N1)pdm09 viruses and patients infected with these viruses in the United States. During 2013–14, fifty-nine (1.2%) of 4,968 analyzed US influenza A(H1N1)pdm09 viruses had the H275Y oseltamivir resistance–conferring neuraminidase substitution. Our results emphasize the need for local surveillance for neuraminidase inhibitor susceptibility among circulating influenza viruses.
During the 2013–14 influenza season, influenza A(H1N1)pdm09 virus was the predominant circulating virus (≈80%) in the United States for the first time since the 2009 pandemic (1). We report and describe characteristics of oseltamivir-resistant influenza A(H1N1)pdm09 viruses and patients infected with these viruses in the United States.
We requested that all US state public health laboratories submit influenza-positive specimens for virologic surveillance, including antiviral susceptibility testing, as described (2). In brief, every 2 weeks each laboratory was asked to send ≤5 specimens for all virus types for virus isolation and neuraminidase (NA) inhibition assay for oseltamivir, zanamivir, and, in a subset, laninamivir and peramivir (3). All oseltamivir-resistant viruses were tested for the H275Y substitution in NA by pyrosequencing (4). Unpropagated influenza A(H1N1)pdm09 virus–positive clinical specimens were screened for the H275Y substitution by pyrosequencing (Technical Appendix). If a cluster (>2 viruses) of oseltamivir-resistant A(H1N1)pdm09 viruses was detected, the state was asked to submit additional influenza A(H1N1)pdm09 virus specimens for testing.
We attempted to collect information by using a standard form from all patients with oseltamivir-resistant virus infection and from a sample of patients with oseltamivir-susceptible virus infection. A 2:1 (susceptible:resistant) sample was randomly selected from the list of tested specimens from the same age group in each state (<5, 5–17, 18–64, and ≥65 years). Patients with oseltamivir-resistant or -susceptible virus infections were compared by using conditional logistic regression models that controlled for age. Full NA and hemagglutinin sequence analysis was performed on all resistant viruses and a subset of susceptible viruses.
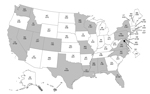
During October 1, 2013–April 30, 2014, a total of 4,968 influenza A(H1N1)pdm09 virus specimens collected from 50 US states and 2 territories were tested for antiviral susceptibility (1,811 virus isolates and 3,157 clinical specimens). A total of 59 (1.2%) influenza A(H1N1)pdm09 viruses from 20 states had the H275Y NA substitution conferring resistance to oseltamivir and peramivir (Figure 1; Table 1). None of 1,811 virus isolates was resistant to zanamivir.
Viruses with the H275Y substitution were detected in patient specimens collected during October 7, 2013–March 25, 2014; monthly prevalence ranged from 0.8% to 2.5%. Among 49 (83.0%) patients with a resistant virus infection and available information, 15 (30.6%) received oseltamivir before specimen collection (Table 2). Prior oseltamivir use was more frequent among hospitalized patients and patients with resistant virus infections than those with susceptible virus infections. Among those with prior exposure, 6 (40.0%) patients with oseltamivir-resistant and none with oseltamivir-susceptible virus infections were immunocompromised (p = 0.03). No differences were found between patients with oseltamivir-resistant or -susceptible virus infections.
Most resistant viruses were clustered in 5 states (California, Hawaii, Louisiana, Mississippi, and Pennsylvania) (Figure 1). Among patients with oseltamivir-resistant virus infection, only 1/4 from California, 0/4 from Hawaii, 3/11 from Louisiana, 1/3 from Mississippi, and 0/14 from Pennsylvania had exposure to oseltamivir before specimen collection. All patients from Pennsylvania except 1 attended 1 of 2 universities (among 7 participating students, none shared classes, residences, or social events). There were no epidemiologic links between other patients. Limited information was available for oseltamivir-treated patients with resistant and susceptible virus infections (Technical Appendix Table).
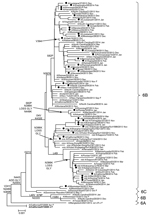
Most hemagglutinin sequences from US influenza A(H1N1)pdm09 viruses collected since October 1, 2013, belonged to the 6B genetic group, and there was minimal separate clustering between susceptible and resistant viruses (Technical Appendix Figure 1). Similar results were observed for the phylogenetic tree of the NA gene (Figure 2). NA sequences from resistant viruses in the United States with the H275Y substitution were generally scattered among other susceptible viruses from genetic group 6B viruses. Most (>99%) influenza A(H1N1)pdm09 viruses currently in circulation have NA substitutions V241I and N369K (Technical Appendix Figure 2). There was >1 cluster of NA sequences with the N386K substitution; each cluster contained susceptible and resistant viruses. Most (>89%) resistant viruses from the United States do not have the N386K mutation.
During the 2013–14 influenza season, prevalence of oseltamivir-resistant influenza A(H1N1)pdm09 viruses was low (≈1%) in the United States, although prevalence was higher in a few states. Most patients infected with an oseltamivir-resistant influenza A(H1N1)pdm09 virus had no prior exposure to oseltamivir. These findings are consistent with a low, and locally variable, level of circulation of resistant viruses. In our study, exposure to oseltamivir before specimen collection was more common among hospitalized patients with resistant virus infections than those with susceptible virus infections. We cannot differentiate whether these viruses emerged during treatment or were present before treatment, but many patients were immunocompromised, a condition associated with emergence of resistance during treatment (5).
Before 2007, resistance to NA inhibitors among influenza viruses circulating globally was low (<1%) (6). However, the 2007–08 influenza showed an emergence of oseltamivir-resistant seasonal influenza A(H1N1) H275Y viruses at variable prevalence (6), and by the 2008–09 season, many countries were reporting up to 100% oseltamivir resistance (7). The sharp increase in seasonal influenza A(H1N1) H275Y viruses from <1% to ≈100% was not attributed to oseltamivir use (8), but was probably caused by evolutionary advantage of H275Y variants. Studies suggest that permissive NA mutations, including R222Q, V234M, and D334N, counteracted the detrimental effect of H275Y on NA function and virus replicative properties, thus enabling virus to remain fully functional (9). The exact mechanism(s) responsible for evolutionary advantage of seasonal influenza A(H1N1) H275Y viruses over oseltamivir-susceptible viruses remain unknown.
Since emergence of influenza A(H1N1)pdm09 virus in 2009, there is concern that the H275Y substitution may become fixed in the viral genome, as it did in seasonal influenza A(H1N1) virus in 2008–09. Oseltamivir-resistance among influenza A(H1N1)pdm09 viruses during their first 2 seasons in circulation (2009–11) remained low (<1%) (2,5). However, during June–August 2011, in Newcastle, New South Wales, Australia, a cluster of oseltamivir-resistant influenza A(H1N1)pdm09 H275Y viruses was detected among patients without prior oseltamivir exposure (10), suggesting community transmission. These H275Y viruses had permissive mutations, V241I and N369K, in addition to N386S (10), which was similar to H275Y viruses isolated in 2012 from Dutch travelers returning from Spain (11). These mutations were believed to offset the destabilizing effect of H275Y and possibly enhance virus transmissibility. The substitutions V241I, N369K, or N386S were not present in influenza A(H1N1)pdm09 virus when it emerged in 2009. However, since 2011, circulating influenza A(H1N1)pdm09 viruses have acquired these substitutions, coinciding with increasing evidence for community transmission of influenza A(H1N1)pdm09 H275Y viruses in the United States and other countries (2,12).
All influenza A(H1N1)pdm09 viruses circulating in the United States in 2013–14 had V241I and N369K substitutions, and ≈10% of resistant viruses and ≈20% of susceptible viruses had an additional substitution (N386K). All influenza A(H1N1)pdm09 H275Y viruses detected in China and Japan in 2013–14 had all 3 substitutions (13). In combination with the H275Y substitution, V241I or N369K enhances surface expression and activity of NA (14). The N386S substitution and the recently detected N386K substitution result in loss of a glycosylation site (15). Although the potential role of these changes in virus spread was suggested (10), no direct evidence is available. Close monitoring for the N386K/S substitution may provide information needed to delineate its role in virus spread. In addition to permissive NA mutations, other properties, such as antigenic novelty, which might provide an advantage to oseltamivir-resistant viruses and facilitate their spread, should also be monitored.
The potential for emergence and spread of oseltamivir-resistant influenza A(H1N1)pdm09 viruses, coupled with limited pharmaceutical options against influenza, emphasizes the need for local surveillance for NA inhibitor susceptibility among circulating influenza viruses. Studies on biologic characteristics (e.g., replication in and transmissibility from ferrets) of influenza A(H1N1)pdm09 virus community isolates with H275Y and other permissive mutations are ongoing.
Dr. Okomo-Adhiambo is a microbiologist in the Virology Surveillance and Diagnosis Branch of the Influenza Division at the Centers for Disease Control and Prevention, Atlanta, Georgia. Her primary research interest is the molecular epidemiology of influenza antiviral drug resistance.
Acknowledgments
We thank our collaborators in US public health laboratories for submitting specimens; colleagues from the Virus Reference Team, Influenza Sequence Activity Team and Molecular Epidemiology Team (Centers for Disease Control and Prevention, Atlanta, GA, USA) for providing valuable assistance; and the 2013–14 US Influenza Antiviral Working Group for providing valuable contributions to US national influenza virologic surveillance.
Members of the 2013–14 US Influenza Antiviral Working Group: Juan A. De La Cruz, Katrina Sleeman, Vasiliy P. Mishin (Influenza Division, Centers for Disease Control and Prevention); Stephanie Chester, Sarah Muir-Paulik, Tricia Aden (Association of Public Health Laboratories, Silver Spring, MD); Catishia Mosley (Alabama Department of Public Health, Montgomery, AL); Cindy Wong, Estela Saguar, Chao-Yang Pan, Nohemi Reyes-Martin, Hugo Guevara, Debra A. Wadford, Erin L. Murray, Ricardo Berumen, Kara Pham, Mahtab Shahkarami, Dongxiang Xia (California Department of Public Health, Richmond, CA); Stacey A. Davis, Vicki Williams, Tanya Martinez (County of San Bernardino Department of Public Health, San Bernadino, CA); Christine L. Wigen, Nicole M. Green, Wendy M. Knight (Los Angeles County Department of Public Health, Los Angeles, CA); Daniel Vigil, Lisa Miller (Colorado Department of Public Health and Environment, Denver, CO); Matthew Pflaum, Myra Ching-Lee, Howard He (Hawaii State Department of Health, Honolulu, HI); Jenna Iberg Johnson, Jose Serrano, and Megan Jespersen (Louisiana Office of Public Health, Baton Rouge, LA); C. Lynn Kane (Allegany County Health Department, Cumberland, MD); Brian Bachaus (Maryland Department of Health and Mental Hygiene, Baltimore, MD); Jennifer Anderson, Paul Byers, Thomas Dobbs, Darlene Bradley, Jane Campbell (Mississippi State Department of Health, Jackson, MS); Anita Valiani (North Carolina Division of Public Health, Raleigh, NC); Amanda Beaudoin, Kirsten Waller (Pennsylvania Department of Health, Harrisburg, PA); Southern Nevada Public Health Laboratory Staff (Las Vegas, NV); Beth Nivin (New York City Department of Health and Mental Hygiene, New York, NY); Kirsten St. George, Jennifer Laplante (Wadsworth Center, New York State Department of Health, Albany, NY); Vickie L. Horan, Michael Zielenski (South Dakota Department of Health, Pierre, SD); Heather Cooks-Sinclair (Austin/Travis County Health and Human Services, Austin, TX); Darrell Irvin, (Beaumont Public Health Department, Beaumont, TX); Steven Hinojosa (Hidalgo County Health and Human Services, Edinburg, TX); Kristin McElroy (Tarrant County Public Health, Fort Worth, TX); Lesley Brannan, LaTasha Martin, Cynthia A. Hernandez (Texas Department of State Health Services, Austin, TX); Diana Thurston, Melanie Spencer (Salt Lake County Health Department, Salt Lake City, UT); Kyle Spackman, Amanda Delgado, Kelly Holmes, Michelle Mendenhall (Unified State Laboratories: Public Health, Taylorsville, UT); Rachelle Boulton (Utah Department of Health, Salt Lake City, UT); April Achter (Virginia Department of Health, Richmond, VA); Soyeon Lippman (Washington Department of Health, Seattle, WA); Erik Reisdorf, Richard Griesser (Wisconsin State Laboratory of Hygiene, Madison, WI); Thomas Haupt (Wisconsin Division of Public Health, Madison, WI); Shannon McBee (West Virginia Bureau for Public Health, Charleston, WV); Reginald McClinton (Wyoming Department of Health, Cheyenne, WY).
References
- Centers for Disease Control and Prevention. Seasonal influenza activity and surveillance [cited 2014 May 21]. http://www.cdc.gov/flu/weekly/
- Storms AD, Gubareva LV, Su S, Wheeling JT, Okomo-Adhiambo M, Pan CY, Oseltamivir-resistant pandemic (H1N1) 2009 virus infections, United States, 2010–11. Emerg Infect Dis. 2012;18:308–11 . DOIPubMedGoogle Scholar
- Okomo-Adhiambo M, Sleeman K, Lysen C, Nguyen HT, Xu X, Li Y, Neuraminidase inhibitor susceptibility surveillance of influenza viruses circulating worldwide during the 2011 Southern Hemisphere season. Influenza Other Respir Viruses. 2013;7:645–58.PubMedGoogle Scholar
- Deyde VM, Sheu TG, Trujillo AA, Okomo-Adhiambo M, Garten R, Klimov AT, Detection of molecular markers of drug resistance in 2009 pandemic influenza A (H1N1) viruses by pyrosequencing. Antimicrob Agents Chemother. 2010;54:1102–10 . DOIPubMedGoogle Scholar
- Graitcer SB, Gubareva L, Kamimoto L, Doshi S, Vandermeer M, Louie J, Characteristics of patients with oseltamivir-resistant pandemic (H1N1) 2009, United States. Emerg Infect Dis. 2011;17:255–7 . DOIPubMedGoogle Scholar
- Sheu TG, Deyde VM, Okomo-Adhiambo M, Garten RJ, Xu X, Bright RA, Surveillance for neuraminidase inhibitor resistance among human influenza A and B viruses circulating worldwide from 2004 to 2008. Antimicrob Agents Chemother. 2008;52:3284–92 . DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Update: influenza activity—United States, September 28, 2008–April 4, 2009, and composition of the 2009–10 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2009;58:369–74 .PubMedGoogle Scholar
- Dharan NJ, Gubareva LV, Klimov AI, Fiore AE, Bresee JS, Fry AM. Antiviral treatment of patients with oseltamivir-resistant and oseltamivir-susceptible seasonal influenza A (H1N1) infection during the 2007–2008 influenza season in the United States. Clin Infect Dis. 2010;50:621–2 . DOIPubMedGoogle Scholar
- Bloom JD, Gong LI, Baltimore D. Permissive secondary mutations enable the evolution of influenza oseltamivir resistance. Science. 2010;328:1272–5 . DOIGoogle Scholar
- Hurt AC, Hardie K, Wilson NJ, Deng YM, Osbourn M, Leang SK, Characteristics of a widespread community cluster of H275Y oseltamivir-resistant A(H1N1)pdm09 influenza in Australia. J Infect Dis. 2012;206:148–57 . DOIGoogle Scholar
- Meijer A, Jonges M, van Beek BP, Swaan CM, Osterhaus AD, Daniels RS, Oseltamivir-resistant influenza A(H1N1)pdm09 virus in Dutch travellers returning from Spain, August 2012. Euro Surveill. 2012;17:20266 .PubMedGoogle Scholar
- Souza TM, Resende PC, Fintelman-Rodrigues N, Gregianini TS, Ikuta N, Fernandes SB, Detection of oseltamivir-resistant pandemic influenza A(H1N1)pdm2009 in Brazil: can community transmission be ruled out? PLoS ONE. 2013;8:e80081 . DOIPubMedGoogle Scholar
- Global Initiative on Sharing All Influenza Data [cited 2014 May 21]. www.gisaid.org
- Butler J, Hooper KA, Petrie S, Lee R, Maurer-Stroh S, Reh L, Estimating the fitness advantage conferred by permissive neuraminidase mutations in recent oseltamivir-resistant A(H1N1)pdm09 influenza viruses. PLoS Pathog. 2014;10:e1004065 . DOIGoogle Scholar
- Sun S, Wang Q, Zhao F, Chen W, Li Z. Glycosylation site alteration in the evolution of influenza A (H1N1) viruses. PLoS ONE. 2011;6:e22844 . DOIGoogle Scholar
Figures
Tables
Cite This Article1Members of the 2013–14 US Antiviral Working Group are listed at the end of this article.
Table of Contents – Volume 21, Number 1—January 2015
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Larisa V. Gubareva, Influenza Division, Centers for Disease Control and Prevention, 1600 Clifton Rd NE, Mailstop G16, Atlanta, GA 30329–4027, USA
Top