Volume 21, Number 10—October 2015
Letter
Familial Trichostrongylus Infection Misdiagnosed as Acute Fascioliasis
To the Editor: Human fascioliasis, infection with Fasciola spp. flukes, is highly pathogenic in both acute and chronic phases and can result in death (1). This disease has been recently emerging, in part linked to climate and global changes (2). Human Fasciola infection has been reported in 5 continents and is related to the disease’s wide spread in livestock. Guilan Province in northern Iran is a fascioliasis-endemic area where the largest human epidemics have occurred, together affecting ≈15,000 persons (3).
In 2014, 3 sisters (ages 35, 33, and 38) and their 41-year-old brother (patients 1–4, respectively) sought medical care at the same time, all with a 3-week history of symptoms. The patients lived in the Langroud district in Guilan Province, at the Caspian Sea littoral; the sisters lived in the same household with their parents, and the brother lived in a nearby household with his wife and son. Patient 1 had mild abdominal and epigastric pain radiating to her back; onset of abdominal pain and flushing during meals; rigors most prominent at night; severe and voluminous diarrhea intensifying after meals; poor appetite; and urticaria associated with itching on her back, chest, and neck. Patient 2 had epigastric and severe right upper quadrant pain that radiated to her back; severe liver tenderness; weakness; nausea; flatulence; continuous diarrhea; urticarial lesions associated with itching on hands, abdomen, and chest; and a history of backache and pulmonary allergy a few months earlier. Patient 3 had abdominal and epigastric pain, loose defecation, and flatulence; urticaria on the neck; and flushing. Patient 4 had abdominal, neck and shoulder pain; constipation; dyspepsia; severe flatulence; and low-grade fever.
Eosinophil levels for patients 1–4, respectively, were 16,260, 2,640, 13,104, and 3,523 cells/mm3. Results from liver function tests were within reference ranges except for lightly increased alanine aminotransferase (35 IU/L) for patient 2. Sonography of liver, pancreas, and spleen showed no abnormalities. Serologic test results for antibodies to Fasciola and Strongyloides were negative. All patients denied close contact with herbivorous animals but mentioned regular consumption of fresh vegetables from local markets or from the parents’ home garden; the latter had been fertilized with sheep manure a few months before symptom onset.
Acute fascioliasis was diagnosed on the basis of symptoms, weight loss, hypereosinophilia, vegetable consumption, and residence in a high-risk area, all typical associations with this illness (1). Absence of fasciolid eggs in stools by Kato-Katz and formalin-ether coprologic methods and lack of sonographic abnormalities were explained by the illness’s early invasive phase, and negative serologic results were explained by the immunologic response heterogeneity in fascioliasis (4) or antigen deterioration. The patients were treated with a single dose of triclabendazole (10 mg/kg).
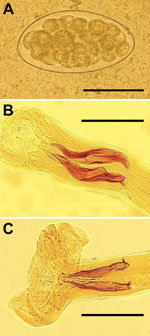
One month after treatment, patients returned with the same symptoms and hypereosinophilia. Unexpectedly, patients 1–3 showed Trichostrongylus eggs in stools (Figure, panel A). The fourth patient’s stool sample was negative. Samples from other family members were analyzed, and 4 (patients’ father and mother and the man’s wife and son) were shedding Trichostrongylus eggs, for a total of 7 patients shedding eggs. T. colubriformis and T. vitrinus nematodes (Figure, panels B and C) were identified in feces 24 hours after treatment. This diagnosis was surprising because, although prevalences as high as 71% for Trichostrongylus spp. in humans have been described in central and southern Iran (5), only sporadic cases have been recently reported.
Infection intensity (24–300 eggs per gram [epg] of feces) correlated with clinical manifestations, indicating light (10–99 epg) to moderate (100–999 epg) severity (6). The patient with 24 epg was asymptomatic, and light or moderate cases are known to be asymptomatic (5). The acute symptomatology in these patients might be explained by their emaciated and weak conditions. Absence of eggs in the initial analyses may be explained by the long prepatent period (4 months–2 years) and by egg shedding discontinuity in light or moderate infections (6–8).
The patients fully recovered, and their eosinophilia returned to reference values <1 month after treatment with 1 dose of albendazole (400 mg), followed by mebendazole (200 mg/day for 3 days). One patient who only partially responded was successfully retreated 1 month later.
Trichostrongyliasis and fascioliasis share many epidemiologic and clinical characteristics. Trichostrongylus spp. infect livestock worldwide, and human infection has been reported in many countries. Eggs are excreted with feces and then hatch and develop into strongyloid larvae. Humans become infected when ingesting these larvae along with vegetables contaminated by animal feces (9). Climate change has been suggested as contributing to the increasing risk for human infection by Trichostrongylus spp. (10). Trichostrongyliasis patients have symptoms like those reported here, although mild eosinophilia may sometimes be the only indication (6–8). Familial outbreaks related to consumption of fresh vegetables fertilized with sheep and goat manure have been reported (6–9).
This familial infection cluster highlights the need to consider trichostrongyliasis in patients with suspected fascioliasis in acute or chronic phases without eggs in stools. This diagnosis is especially possible if patients have consumed fresh vegetables fertilized with fresh livestock manure or have had close contact with herbivorous animals.
References
- Mas-Coma S, Agramunt VH, Valero MA. Neurological and ocular fascioliasis in humans. Adv Parasitol. 2014;84:27–149. DOIPubMedGoogle Scholar
- Afshan K, Fortes-Lima CA, Artigas P, Valero MA, Qayyum M, Mas-Coma S. Impact of climate change and man-made irrigation systems on the transmission risk, long-term trend and seasonality of human and animal fascioliasis in Pakistan. Geospat Health. 2014;8:317–34. DOIPubMedGoogle Scholar
- Ashrafi K, Valero MA, Massoud J, Sobhani AR, Solaymani-Mohammadi S, Conde P, Plant-borne human contamination by fascioliasis. Am J Trop Med Hyg. 2006;75:295–302.PubMedGoogle Scholar
- Mas-Coma S, Bargues MD, Valero MA. Diagnosis of human fascioliasis by stool and blood techniques: update for the present global scenario. Parasitology. 2014;141:1918–46. DOIPubMedGoogle Scholar
- Ghadirian E, Arfaa F. Present status of trichostrongyliasis in Iran. Am J Trop Med Hyg. 1975;24:935–41.PubMedGoogle Scholar
- Wolfe MS. Oxyuris, Trichostrongylus and Trichuris. Clin Gastroenterol. 1978;7:201–17.PubMedGoogle Scholar
- Boreham RE, McCowan MJ, Ryan AE, Allworth AM, Robson JM. Human trichostrongyliasis in Queensland. Pathology. 1995;27:182–5. DOIPubMedGoogle Scholar
- Ralph A, O’Sullivan MV, Sangster NC, Walker JC. Abdominal pain and eosinophilia in suburban goat keepers—trichostrongylosis. Med J Aust. 2006;184:467–9.PubMedGoogle Scholar
- Lattes S, Ferte H, Delaunay P, Depaquit J, Vassallo M, Vittier M, Trichostrongylus colubriformis nematode infections in humans, France. Emerg Infect Dis. 2011;17:1301–2. DOIPubMedGoogle Scholar
- Mas-Coma S, Valero MA, Bargues MD. Effects of climate change on animal and zoonotic helminthiases. Rev Sci Tech (OIE). 2008;27:443–57.PubMedGoogle Scholar
Figure
Cite This ArticleRelated Links
Table of Contents – Volume 21, Number 10—October 2015
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Santiago Mas-Coma, Departamento de Parasitologia, Facultad de Farmacia, Universidad de Valencia, Av Vicent Andres Estelles s/n, 46100 Burjassot, Valencia, Spain
Top