Volume 21, Number 8—August 2015
Dispatch
Cutaneous Legionella longbeachae Infection in Immunosuppressed Woman, United Kingdom
Cite This Article
Citation for Media
Abstract
We report a rare case of cutaneous Legionella longbeachae infection in a patient receiving long-term corticosteroids for immune thrombocytopenia. Such infections cannot be identified by using Legionella urinary antigen testing but are commonly seen after exposure to commercial potting compost, particularly in immunocompromised patients.
A 70-year-old woman in whom stage A0 chronic lymphocytic leukemia had been diagnosed in 2004 by immunophenotyping was monitored with a watch-and-wait strategy for 8 years until she started treatment for anemia and splenomegaly. She was given 4 cycles of fludarabine, cyclophosphamide, and rituximab until her illness went into clinical remission.
Twelve months later, the woman sought treatment for extensive purpura. She had a platelet count of 1 × 109/mL (reference range 150–400 × 109/mL) but an otherwise unremarkable complete blood count, peripheral blood smear, and bone marrow aspirate and trephine biopsy result. Immune thrombocytopenia was diagnosed, and 1 mg/kg of oral prednisolone daily was initiated, to which the illness responded well. Over 8 weeks, steroids were weaned to 20 mg once daily. During this time, the woman had a colonoscopy to investigate 6 weeks of persistent diarrhea. Cultures of fecal samples and multiple colonic biopsies were unremarkable.
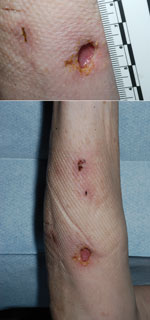
During a routine outpatient review in August 2013, three discrete erythematous nodules were found on the ventral surface of her right forearm. The lesions were tender and nonpurulent, contained no punctum, and were distinct and separate from the site where an intravenous cannula had been placed for her colonoscopy. She had no history of tick or animal bite, recent foreign travel, or trauma. Her diarrheal illness had resolved before this review. Cellulitis was presumptively diagnosed, and the woman was started on a course of oral flucloxacillin.
Two weeks later, she was admitted to the hospital with worsening pain and swelling of the right forearm. She remained systemically well. Laboratory testing indicated a leukocyte count of 9.6 × 109 cells/L (reference range 4.0–11.0 × 109 cells/L), with neutrophils 8.7 × 109 cells/L (reference range 1.8–7.5 × 109 cells/L), lymphocytes 0.4 × 109 cells/L (reference range 1.5–4.0 × 109 cells/L), monocytes 0.4 × 109 cells/L (reference range 0.2–1.0 × 109 cells/L), eosinophils 0.1 × 109 cells/L (reference range 0–0.4 × 109 cells/L), and basophils 0 × 109 cells/L (reference range 0–0.4 × 109 cells/L). C-reactive protein level was 17 mg/L. She was treated empirically with intravenous flucloxacillin.
Initial blood culture results were negative. Cultures of wound swab samples were negative for methicillin-resistant Staphylococcus aureus but showed evidence of gram-negative bacilli. Results from urinary Legionella and pneumococcal antigen tests were negative. Surgical review prompted incision and drainage of the lesions, the largest of which was 4 cm × 5 cm (Figure). Histopathologic examination of the lesions showed only granulation tissues; no malignant cells were seen.
Lack of clinical improvement prompted a change in antimicrobial drug therapy to intravenous tazocin and clindamycin. Results from serial bacterial cultures of blood, feces, and tissue failed to yield further positive results. Results from PCR for herpes simplex virus types 1 and 2 and varicella zoster virus were negative. Serum β-D-glucan was elevated at 262 pg/mL (reference range <80 pg/mL); however, the patient did not receive antifungal treatment because of the lack of clinical suspicion of fungal infection and the test’s high false-positive rate.
A dermatology review suggested that the lesions represented a sporotrichoid lymphocutaneous infection, possibly caused by an atypical mycobacterium. Results from an extended culture for mycobacterium, auramine stains for acid-fast bacilli, and further histopathologic staining for fungal hyphae were negative. Despite antimicrobial drug therapy, the lesions persisted for 2 weeks; repeat incision and drainage was performed.
A pus sample was sent to Great Ormond Street Hospital (London, UK) for PCR. Species-specific real-time PCR for S. aureus and S. pyogenes were negative. Subsequent analysis by broad-range 16S rDNA PCR (1) gave a strongly positive result, and the amplicon sequence exactly matched 16S rDNA sequences from 2 L. longbeachae–type strains, leading to a diagnosis of cutaneous infection with L. longbeachae.
The woman was started on a 6-week course of triple antimicrobial drug therapy with ciprofloxacin, azithromycin, and rifampin. By her 3-month follow-up, her lesions had resolved completely. Although she was not a keen gardener, she reported that, a week before her lesions appeared, she had handled a leaking potted plant without thoroughly washing her hands afterward.
Legionella longbeachae was first described in Long Beach, California, USA, in 1981; it was isolated from respiratory tract specimens from 4 patients with pneumonia (2). A second serogroup was identified later that year (3). Reported cases of L. longbeachae infection are rare in Europe. However, in New Zealand, Australia, and Japan, they are as common as infections with L. pneumophila and often cause legionellosis and Pontiac fever, a nonpneumonic, self-limiting illness characterized by influenza-like symptoms (4,5). Only testing of urinary antigens can identify L. pneumophila serogroup 1, whereas L. longbeachae can be detected by serologic testing, culture, or PCR (6).
Unlike infections with L. pneumophila, which have been linked to water systems in the built (i.e., human-created) environment, infections with L. longbeachae are most commonly associated with the use of commercial potting compost (4,7,8). L. longbeachae was first isolated from potting mix after an outbreak of infections in South Australia in 1989 (9). Since then, outbreaks have been linked to the use of potting compost in Australia, New Zealand, Japan, the Netherlands, and most recently Scotland, where 4 cases of L. longbeachae infection were confirmed during 2008–2009 (4,5,7,10). A 2013 study of compost in the United Kingdom found that 15 of the 24 samples tested contained Legionella species, of which 4 were identified as L. longbeachae serogroup 1 (11).
A 2006 case–control study (7) showed that risk factors that predicted L. longbeachae infection included poor hand hygiene after gardening and proximity to dripping hanging flower pots, the latter indicating that ingestion might be an alternate possible route of transmission to aerosolization (4,7). These risk factors suggest a possible route of infection for the patient reported here; she reported handling a large, leaking potted plant and admitted to poor hand hygiene before meals. However, L. longbeachae cannot be confirmed as the source of her infection because her potting soil was not tested. An alternative route of infection would be through the site of the cannula that was inserted for her colonoscopy. Other reported risk factors include smoking, preexisting respiratory disease, and immunosuppression (4,6,7,12). The primary host defense mechanism in L. longbeachae infection is cell-mediated immunity, depression of which through the use of corticosteroids or immunosuppressive drugs may predispose patients to legionellosis. A 2007 report describes a case of L. longbeachae pneumonia after corticosteroid therapy for chronic immune thrombocytopenia (6).
The clinical picture in L. longbeachae infection is typically similar to that of infection with L. pneumophila; however, as in this case, variations have been reported. A 2012 case report described a patient in whom L. longbeachae endocarditis developed 6 months after a bioprosthetic aortic valve replacement (13). Several cases of cutaneous infection secondary to Legionella species have been reported (14); 7 of the 13 confirmed cases occurred in immunocompromised patients. Because so many cases occurred in immunocompromised patients, we recommend use of broad-range 16S rDNA PCR to detect L. longbeachae in immunosuppressed patients with respiratory or influenza-like symptoms who report a history of exposure to commercial potting compost.
Dr. Grimstead is a Foundation Programme doctor at Torbay Hospital, South Devon Healthcare Foundation Trust. He has a special interest in hematology.
References
- Harris KA, Hartley JC. Development of broad range 16S rDNA PCR for use in the routine clinical microbiology service. J Med Microbiol. 2003;52:685–91. DOIPubMedGoogle Scholar
- McKinney RM, Porschen RK, Edelstein PH, Bissett ML, Harris PP, Bondell SP, Legionella longbeachae species nova, another etiologic agent of human pneumonia. Ann Intern Med. 1981;94:739–43. DOIPubMedGoogle Scholar
- Bibb WF, Sorg RJ, Thomason BM, Hicklin MD, Steigerwalt AG, Brenner DJ, Recognition of a second serogroup of Legionella longbeachae. J Clin Microbiol. 1981;14:674–7.PubMedGoogle Scholar
- Whiley H, Bentham R. Legionella longbeachae and legionellosis. Emerg Infect Dis. 2011;17:579–83. DOIPubMedGoogle Scholar
- Cramp GJ, Harte D, Douglas NM, Graham F, Schousboe M, Sykes K. An outbreak of Pontiac fever due to Legionella longbeachae serogroup 2 found in potting mix in a horticultural nursery in New Zealand. Epidemiol Infect. 2010;138:15–20. DOIPubMedGoogle Scholar
- Kümpers P, Tiede A, Kirschner P, Girke J, Ganser A, Peest D. Legionnaires’ disease in immunocompromised patients: a case report of Legionella longbeachae pneumonia and review of the literature. J Med Microbiol. 2008;57:384–7. DOIPubMedGoogle Scholar
- O’Connor BA, Carman J, Eckert K, Tucker G, Givney R, Cameron S. Does using potting mix make you sick? Results from a Legionella longbeachae case–control study in South Australia. Epidemiol Infect. 2007;135:34–9. DOIPubMedGoogle Scholar
- Lindsay DS, Brown AW, Brown DJ, Pravinkumar SJ, Anderson E, Edwards GF. Legionella longbeachae serogroup 1 infections linked to potting compost. J Med Microbiol. 2012;61:218–22. DOIPubMedGoogle Scholar
- Steele TW, Moore CV, Sangster N. Distribution of Legionella longbeachae serogroup 1 and other legionellae in potting soils in Australia. Appl Environ Microbiol. 1990;56:2984–8.PubMedGoogle Scholar
- Pravinkumar SJ, Edwards G, Lindsay D, Redmond S, Stirling J, House R, A cluster of Legionnaires’ disease caused by Legionella longbeachae linked to potting compost in Scotland, 2008–2009. Euro Surveill. 2010;15:19496.PubMedGoogle Scholar
- Currie SL, Beattie TK, Knapp CW, Lindsay DS. Legionella spp. in UK composts—a potential public health issue? Clin Microbiol Infect. 2014;20:O224–9. PubMedGoogle Scholar
- Amodeo MR, Murdoch DR, Pithie AD. Legionnaires’ disease caused by Legionella longbeachae and Legionella pneumophila: comparison of clinical features, host-related risk factors, and outcomes. Clin Microbiol Infect. 2010;16:1405–7. DOIPubMedGoogle Scholar
- Leggieri N, Gouriet F, Thuny F, Habib G, Raoult D, Casalta JP. Legionella longbeachae and endocarditis. Emerg Infect Dis. 2012;18:95–7. DOIPubMedGoogle Scholar
- Padrnos LJ, Blair JE, Kusne S, DiCaudo DJ, Mikhael JR. Cutaneous legionellosis: case report and review of the medical literature. Transpl Infect Dis. 2014;16:307–14. DOIPubMedGoogle Scholar
Figure
Cite This ArticleTable of Contents – Volume 21, Number 8—August 2015
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Daniel Grimstead, Haematology Department, Torbay Hospital, South Devon Healthcare Trust, Lawes Bridge, Torquay, Devon, TQ2 7AA, UK
Top