Volume 21, Number 8—August 2015
Research
Risk for Mycobacterial Disease among Patients with Rheumatoid Arthritis, Taiwan, 2001–2011
Abstract
Increasing evidence indicates that the risk of acquiring tuberculosis (TB) and nontuberculous mycobacterial disease is elevated among patients with rheumatoid arthritis (RA). To determine the epidemiology of mycobacterial diseases among RA patients in Asia, we conducted a retrospective cohort study. We used a nationwide database to investigate the association of RA with mycobacterial diseases. The risk for development of TB or nontuberculous mycobacterial disease was 2.28-fold and 6.24-fold higher among RA patients than among the general population, respectively. Among RA patients, risk for development of mycobacterial disease was higher among those who were older, male, or both. Furthermore, among RA patients with mycobacterial infections, the risk for death was increased. Therefore, RA patients, especially those with other risk factors, should be closely monitored for development of mycobacterial disease.
The major clinical spectrum of mycobacterial diseases is caused by tuberculosis (TB) and nontuberculous mycobacteria (NTM). TB remains a major global health problem; in 2012, an estimated 8.6 million persons became infected and 1.3 million died of the disease (1). NTM are ubiquitous environmental microorganisms that cause chronic pulmonary and extrapulmonary infection in patients with inflammatory diseases (2). Several NTM strains are resistant to many antimicrobial drugs, making treatment difficult (3). Because reporting of NTM disease to public health administrations is not required in most countries, epidemiologic data for these countries are not available (4). Pulmonary diseases caused by NTM are being diagnosed with increasing frequency worldwide (5), including in Taiwan (6). In Taiwan, the incidence of TB remains high, despite extensive implementation of well-known TB control measures and use of the Bacillus Calmette-Guérin vaccine (7); between 2000 and 2012, a laboratory-based study indicated a trend of decreasing TB cases but significantly increasing NTM cases in Taiwan (6).
Rheumatoid arthritis (RA), a chronic articular inflammatory disease (8), affects 0.5%–1.0% of the adult population and is a major cause of disability in industrialized countries (9,10). Among RA patients, the risks of acquiring or dying of an infectious disease are increased, possibly because of disease-related immune dysfunction or the immunosuppressive effects of therapeutic agents (11). In Europe and the United States, an increased risk for TB among RA patients has been reported (12), and the risk for active TB is even higher among those receiving anti–tumor necrosis factor α (TNF-α) therapy (13). Previous clinical studies have shown that the prevalence of latent tuberculosis infection was higher among RA patients than among healthy controls (14). A recent study indicated that in the United States, the incidence of NTM disease was significantly higher among RA patients receiving anti–TNF-α therapy than among patients with other inflammatory diseases who were receiving the same treatment (15). The prevalence of mycobacterial diseases is higher among the general population in Asia than in the United States and Europe (1,16). However, few population-based epidemiologic studies have investigated the association of RA with mycobacterial diseases in Asia. In addition, prevalence of concurrent medical conditions is higher among RA patients (17,18), which may affect their risk for TB (19). However, the association of RA with concurrent medical conditions and mycobacterial infection is unclear.
In Taiwan, the National Health Insurance program is a mandatory universal health insurance program that provides comprehensive medical care for >99% of Taiwan’s residents (20–22). The National Health Insurance Research Database (NHIRD) is managed by the National Health Research Institutes, and confidentiality is maintained according to Bureau of National Health Insurance guidelines (23). We used this nationwide database to conduct a retrospective cohort study investigating the association between RA and mycobacterial diseases in Taiwan during 2001–2011.
Data Source
The NHIRD includes inpatient and ambulatory care claims covering 1996–2011. The Longitudinal Health Insurance Database 2000 contains all original claims data for 1 million persons randomly sampled from the Registry for Beneficiaries of the NHIRD, which was released by the National Health Research Institutes, which confirmed that the random samples were representative of the general population in Taiwan. We systemically sampled NHIRD patient data for 2001–2011 (Figure 1). The data were de-identified forms of secondary information in an anonymous format released to the public for research purposes. All work was done at the Taichung Veterans General Hospital, Taichung, Taiwan, and the institutional review board of this hospital exempted this study from full review (no. CE13151-1).
Definitions
Patients with different diseases were identified by use of codes from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). In addition, the NHIRD includes a registry system for catastrophic illnesses, and RA is included in this database. Therefore, the diagnosis of RA (ICD-9-CM code 714.0) was made according to the 1987 American College of Rheumatology criteria (24) and the NHIRD Registry of Catastrophic Illness Patient Database.
All TB and NTM cases were determined by use of ICD-9-CM codes, laboratory mycobacterium examination codes, and antimycobacterial therapy receipts; all 3 criteria needed to be met. We used the definition of TB in the published literature (20,21). First, the ICD-9-CM codes for TB were 010–018. Among TB cases, ICD-9-CM codes 010–012 and 018 are used for pulmonary TB and codes 013–017 are used for extrapulmonary TB. Second, >2 mycobacteria laboratory examination codes were used for TB identification, including those identified by acid-fast smear (13006C/13025C), mycobacterial culture (13012C/13026C), acid-fast bacillus differentiation (13013C), tuberculin test (12106C), tuberculosis test (13024C), and bronchoscopy (28006C) results (20). Third, we identified patients who had taken at least 1 prescription consisting of >3 anti-TB drugs used simultaneously for at least 120 days during a 180-day period; these drugs were isoniazid, ethambutol, rifampin, pyrazinamide, amikacin, kanamycin, streptomycin, ciprofloxacin, ofloxacin, moxifloxacin, levofloxacin, prothionamide, clarithromycin, and thioridazine (20,21).
The NTM disease definition was based on the American Thoracic Society and the Infectious Diseases Society of America guidelines (3), which include clinical, radiographic, and microbiologic criteria. All items carry equal weight and all must be met for a diagnosis of NTM lung disease (3) as follows. First, the patient must have been assigned an ICD-9-CM code for NTM disease (031.0, 031.1, 031.2, 031.8, and 031.9). Among NTM diseases, ICD-9-CM code 031.0 is for pulmonary NTM disease and the others are for extrapulmonary NTM disease. Second, the mycobacteria laboratory examination code criteria for NTM needed to be consistent with those for TB (20). Third, the patient should have received >2 drugs to treat NTM disease, including amikacin, cefoxitin, ciprofloxacin, clarithromycin, doxycycline, ethambutol, imipenem, levofloxacin, meropenem, minocycline, moxifloxacin, rifabutin, rifampin, tigecycline, and streptomycin (3). Simultaneous TB and NTM disease was defined as TB only. In addition, the first diagnosis of TB or NTM disease must have been made >6 months after the identification of the RA. The study end point was defined as the onset of new mycobacterial disease or death during the 11-year follow-up period (2001–2011).
To understand the association between RA-related concurrent conditions and mycobacterial diseases, we choose several RA-related conditions to study. The definitions of each condition were based on the following ICD-9-CM codes: cardiovascular diseases (codes 390–438), diabetes mellitus (code 250), hyperlipidemia (code 272), liver cirrhosis (code 571), and chronic obstructive pulmonary disease (codes 490–492 and 496). In addition, we also selected mycobacterial disease-related conditions (e.g., chronic kidney disease [code 585] and HIV disease [codes 042–044]).
Study Population
The study was a retrospective cohort study of NHIRD data for 29,746 patients >18 years of age who had received a new diagnosis of RA during January 1, 2001, through December 31, 2011. We excluded 749 patients whose TB or NTM diagnosis was made within 180 days after the RA diagnosis and/or who died within 180 days after RA diagnosis. The age-matched control group (>18 years of age) was selected from the NHIRD Longitudinal Health Insurance Database 2000 and excluded patients for whom information regarding age or sex was missing, those with a history of RA, TB, or NTM, and those who died before July 1, 2001. Patients with newly diagnosed RA (28,997 patients) and controls who did not have RA (115,988 controls) were matched 1:4 by age and sex (Figure 1).
Statistical Analyses
Data are presented as mean ± SD for continuous variables and as proportions for categorical variables. Differences were analyzed by using the independent t-test for the continuous variables and the χ2 test for the categorical variables. Incidence rates for newly diagnosed mycobacterial diseases among RA patients and controls were calculated. For each cohort, incidence rates per 1,000 person-years were also calculated according to the distribution of demographic characteristics and concurrent conditions. The multivariable Cox proportional hazards model was adjusted for age, sex, Charlson Comorbidity Index score (25), and urbanization (residence in urban, suburban, or rural area) before being used to identify independent factors contributing to the development of mycobacterial diseases in the RA and control cohorts. The 95% CI for each variable was also determined. All analyses were conducted by using SAS statistical software version 9.3 (SAS Institute, Inc., Cary, NC, USA). A p value of <0.05 was considered significant.
Characteristics of the Study Cohort
Data for ≈99% of Taiwan residents were included in the NHIRD. Among these, 28,997 eligible persons with newly diagnosed RA were identified during 2001–2011. The follow-up period for this study population, during which risk for mycobacterial disease development was assessed; mean follow-up time was 5.1 years (range 2.1–8.3 years). The control group was matched for age and sex at a ratio of 1:4, and a total of 115,988 participants who did not have RA (controls) were identified (Figure 1). Characteristics of the enrolled participants are summarized in Table 1. RA patients were predominately (77.7%) female, and mean age was 53.9 years. Approximately 22.9% of the RA patients were >65 years of age. Age and sex distributions between the RA patients and controls did not differ significantly. Prevalence of concurrent conditions was higher among RA patients than among controls (56.4% vs. 32.1%, respectively; p<0.0001). No clinically significant difference in urbanization was found between RA patients and controls.
Characteristics of RA Patients with Mycobacterial Disease
Among the total study population of 144,985, new-onset TB developed in 2,144 (1.48%) (Figure 1). Among the 28,997 RA patients, 518 (1.79%) received a new diagnosis of TB at least 6 months after their RA diagnosis (Figure 1). Of the 518 patients with TB that we identified, the TB was pulmonary for 457 (88.22%) and extrapulmonary for 61 (11.78%). The incidence rate for TB was higher among RA patients than controls (3.4 vs. 1.4 cases/1,000 person-years, respectively; Table 2). After multivariable analysis and adjustment for age, sex, Charlson Comorbidity Index score, and urbanization, the risk for TB development increased 2.28-fold for RA patients over that for controls (95% CI 2.06–2.54; p<0.0001). The risk for TB development, after stratification for age, sex, and urbanization, was higher for RA patients than controls. Further analysis indicated that the risk for TB development was higher for RA patients with concurrent conditions than for controls with the same conditions.
New-onset NTM disease developed in 131 (0.09%) patients (Figure 1). For 52 (0.18%) of the 28,997 RA patients, NTM disease was newly diagnosed at least 6 months after RA was diagnosed (Figure 1). Of these 52 patients with identified NTM, 27 (51.92%) had pulmonary and 25 (48.08%) had extrapulmonary disease; 27 (51.92%) had a history of previous TB. The incidence rate of NTM disease was significantly higher among RA patients than controls (0.3 vs. 0.1 cases/1,000 person-years, respectively; Table 2). Multivariable analysis indicated that risk for development of NTM disease was 6.24-fold higher among RA patients than controls (95% CI 4.24–9.17, p<0.0001). As was the case for TB, the risk for development of NTM disease after stratification for age, sex, and urbanization was higher for RA patients than controls. In addition, risk for development of NTM disease was higher for RA patients with concurrent conditions than for controls with the same conditions.
Risk Factors for Mycobacterial Disease Development
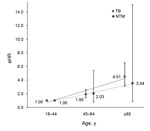
Risk factors for development of mycobacterial diseases in RA patients were older age and male sex. For patient age >65 years, the adjusted hazard ratio [aHR] was 5.06 (95% CI 3.72–6.88; p<0.0001) for TB and 4.70 (95% CI 1.82–12.13; p = 0.001) for NTM. For male patients, aHR was 2.35 (95% CI 1.97–2.81; p<0.0001) for TB and 2.64 (95% CI 1.52–4.60; p = 0.001) for NTM (Table 3). In addition, among RA patients, the risk for TB and NTM was positively correlated with increased age (Figure 2). No significant difference in urbanization was found for TB and NTM disease development in RA patients.
Risk for Death among RA Patients with Mycobacterial Disease
Risk for death was higher among RA patients with mycobacterial infection than among RA patients without mycobacterial infection (aHR 1.62, 95% CI 1.38–1.90, p<0.0001 for TB; aHR 3.06, 95% CI 1.53–6.12, p = 0.002 for NTM; Table 4). Furthermore, the risk for death was increased among older RA patients (>65 years, aHR 8.81, 95% CI 7.48–10.39; p<0.0001), male patients (aHR 1.73, 95% CI 1.59–1.88; p<0.0001), and patients who lived in rural areas (aHR 1.41, 95% CI 1.29–1.54; p<0.0001).
This nationwide population-based retrospective cohort study indicated that among the ≈20 million enrollees in Taiwan’s NHIRD, the risk for development of TB was 2.28-fold greater and the risk for development of NTM disease 6.24-fold greater among RA patients than among controls. Among RA patients, significant risk factors for development of mycobacterial disease were older age (>65 years) and male sex. Furthermore, the risk for death was higher among RA patients with mycobacterial infection than among RA patients without mycobacterial infection.
The incidence of TB (53.0 cases/100,000 population in 2012) and mortality rate (2.7 cases 100,000 population in 2012) were higher in Taiwan than in other Asian (e.g., Japan, Korea) or western countries (7). In this study, the incidence rate for TB was significantly higher among RA patients than controls (3.4 vs. 1.4/1,000 person-years, respectively). A population-based study in Europe showed that the risk for development of TB was 4-fold higher among RA patients than among the general population (26). Although the incidence rate for TB for the general population declined over time, the incidence rate for TB among RA patients was still higher than that among controls (26).
Our findings that male sex and older age are major risk factors for mycobacterial infection among RA patients in Taiwan are consistent with local laboratory-based data (6) and findings of systematic reviews in Asia (16,27). However, in Japan, South Korea, and most western countries, most NTM disease patients were female (16,28). This discrepancy may be associated with lifestyle, environmental factors, and genetic factors. More studies are required to confirm this hypothesis. We also found a history of TB for 27 (51.92%) of NTM-infected RA patients and 43 (54.43%) of 79 controls, similar to results from eastern Asia (27). Furthermore, our results illustrated that risk for development of NTM disease was 6.24-fold higher among RA patients than controls and that the risk for death among RA patients with NTM disease was 3.06-fold higher than that for RA patients without NTM disease. A recent population-based study in Canada demonstrated a 2.07-fold increased risk for NTM disease among RA patients and a 1.81-fold increased risk for death among RA patients with NTM disease (29). The risk for exposure to mycobacteria closely correlated with the prevalence of mycobacterial diseases. The prevalence of mycobacterial diseases is higher in Taiwan (5,7), thus leading to an increased risk for infection (aHRs 6.24 vs. 2.07) and infection-related death (aHRs 3.06 vs. 1.81) among RA patients in Taiwan and Canada, respectively.
Among participants in this study, the risk for death among RA patients with NTM disease was higher than that among RA patients TB (aHRs 3.06 vs.1.62). This finding might reflect increased incidence of NTM disease in Taiwan, but awareness of NTM disease is not enough. In Taiwan, cases of TB should be reported to the public health administration, and control measures should be implemented immediately. However, cases of NTM disease do not have to be reported, and few hospitals can fully identify NTM (6). Furthermore, the severity of NTM disease varies, resulting in missed diagnoses for patients with mild and asymptomatic infection. Therefore, it is easy to ignore and underestimate the consequences of NTM disease. According to experience in Europe (26), risk for mycobacterial diseases in RA patients is reduced by increasing the awareness of hospital staff and screening for mycobacterial diseases. In addition, more laboratory data are needed for investigation of the characteristics (e.g., species distribution, invasion site, drug resistant) of clinical NTM strains.
In this study, pulmonary invasion affected most RA patients (88.22%) and controls (92.50%) with TB and slightly more than half (51.92%) of the RA patients with NTM disease. In the past few years, the incidence of NTM-associated pulmonary diseases and hospitalization has increased in Europe and the United States (30,31), and this increase is most likely associated with the global trend toward more persons being of older age, increased use of immunosuppressive medication, elevated prevalence of immune-modulating concurrent conditions, and use of advanced diagnostic techniques (16). In addition to pulmonary NTM disease, an increased percentage of extrapulmonary NTM disease was noted in RA patients than controls (48.08% vs. 34.18%). Similar elevated manifestations of extrapulmonary NTM (44%) were reported from another study, which found NTM disease in RA patients receiving anti–TNF-α therapy in the United States (32,33).
Our results showed that the risk for development of mycobacterial diseases was higher among RA patients with concurrent medical conditions than among controls with the same conditions. These findings were similar to those of a population-based study in Canada (29). Accumulating evidence indicates that risk factors for TB are diabetes mellitus, liver cirrhosis, and chronic obstructive pulmonary disease (19). However, the relationship between other RA-related conditions (e.g., hyperlipidemia) and mycobacterial diseases remains unclear. In addition to demographic and host factors, geographic factors are associated with the development of mycobacterial diseases (19,34). A population-based study in the United States found that TB-related deaths were strongly predominant among the general population living in urban areas, but no such meaningful difference was found for NTM disease–related deaths (34). Our results showed that after stratification of data for urbanization, the risk for both TB and NTM disease among all RA patients was higher than that for controls, but no significant difference with regard to urbanization and mycobacterial disease development in RA patients was found. Urbanization had fewer effects on increasing the risk for development of mycobacterial diseases in RA patients in Taiwan.
The NHIRD contains complete medical information regarding patients, including prescription details, diagnoses based on the ICD-9-CM codes, registration files, and original claims records for reimbursement. However, this administrative database has several limitations. First, the NHIRD does not contain detailed information of lifestyle factors (e.g., smoking, alcohol abuse, or substance abuse) or individual health status (e.g., body mass index, malnutrition, genetic factors) that are associated with mycobacterial infection and RA (19,35). Another limitation is that the NHIRD only contains medical claims data, so the current condition of the patients is unknown. Of note, the NHIRD does not include the results of laboratory examinations. To improve accuracy of the analysis, we used not only ICD-9-CM codes but also mycobacteria laboratory examination codes and antimycobacterial therapy receipts to identify TB and NTM disease cases. Because most patients with NTM pulmonary lung disease do not receive treatment, our definition (which requires treatment) is probably relatively insensitive, missing all untreated cases. The definition is undoubtedly highly specific, but because extrapulmonary NTM is almost always treated, our definition probably resulted in a higher than expected proportion of extrapulmonary NTM cases. The major strength of this study is use of a nationwide database with medical care records, which is minimally affected by selection and recall biases. In addition, the large sample size of the NHIRD (≈20 million enrollees, including patients and the general population) and long-term records enhanced the statistical power and accuracy of this study.
Increasing evidence indicates that the elevated risk for mycobacterial diseases among RA patients is primarily attributable to the effect of immunosuppressive therapies (15,32,33). Because most RA patients in Taiwan were treated with disease-modifying antirheumatic drugs or biologicals, we hypothesized that the risk for development of mycobacterial diseases may be associated with immunosuppressive therapy, RA-related concurrent conditions, or immune dysfunction caused by RA itself. Further studies are required to confirm this hypothesis.
In conclusion, our results showed that risk for death is higher among RA patients with than without mycobacterial diseases. Therefore, TB- or NTM-infected RA patients should be closely monitored, especially those who are male, are >65 years of age, or have concurrent conditions.
Dr. Liao is an associate research fellow at Taichung Veterans General Hospital, Taiwan. Her primary research interest is infectious diseases, especially TB and NTM diseases.
Acknowledgments
We thank the Healthcare Service Research Center of Taichung Veterans General Hospital for statistical support.
This study was supported in part by grants from Taichung Veterans General Hospital, Taiwan (TCVGH-NHRI10307). This study is based in part on data from the NHIRD provided by the National Health Insurance Administration, Ministry of Health and Welfare and managed by National Health Research Institutes (registered no. 101095, 102148).
References
- World Health Organization G. Global tuberculosis report 2013 [cited 2014 Nov 15]. http://www.who.int/tb/publications/global_report/en/
- Falkinham JO. Impact of human activities on the ecology of nontuberculous mycobacteria. Future Microbiol. 2010;5:951–60 . DOIPubMedGoogle Scholar
- Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. DOIPubMedGoogle Scholar
- Thomson RM. Changing epidemiology of pulmonary nontuberculous mycobacteria infections. Emerg Infect Dis. 2010;16:1576–83. DOIPubMedGoogle Scholar
- Brode SK, Daley CL, Marras TK. The epidemiologic relationship between tuberculosis and non-tuberculous mycobacterial disease: a systematic review. Int J Tuberc Lung Dis. 2014;18:1370–7. DOIPubMedGoogle Scholar
- Chien JY, Lai CC, Sheng WH, Yu CJ, Hsueh PR. Pulmonary infection and colonization with nontuberculous mycobacteria, Taiwan, 2000–2012. Emerg Infect Dis. 2014;20:1382–5. DOIPubMedGoogle Scholar
- Centers for Disease Control. R.O.C. (Taiwan). Statistics of communicable diseases and surveillance report 2012. Taipei City (Taiwan): The Centers; 2013.
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. DOIPubMedGoogle Scholar
- Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15–25. DOIPubMedGoogle Scholar
- Lee J, Dunlop D, Ehrlich-Jones L, Semanik P, Song J, Manheim L, Public health impact of risk factors for physical inactivity in adults with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:488–93. DOIPubMedGoogle Scholar
- Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287–93. DOIPubMedGoogle Scholar
- Carmona L, Hernandez-Garcia C, Vadillo C, Pato E, Balsa A, Gonzalez-Alvaro I, Increased risk of tuberculosis in patients with rheumatoid arthritis. J Rheumatol. 2003;30:1436–9 .PubMedGoogle Scholar
- Keane J. TNF-blocking agents and tuberculosis: new drugs illuminate an old topic. Rheumatology (Oxford). 2005;44:714–20. DOIPubMedGoogle Scholar
- Chen DY, Shen GH, Chen YM, Chen HH, Hsieh CW, Lan JL. Biphasic emergence of active tuberculosis in rheumatoid arthritis patients receiving TNFα inhibitors: the utility of IFNγ assay. Ann Rheum Dis. 2012;71:231–7. DOIPubMedGoogle Scholar
- Winthrop KL, Baxter R, Liu L, Varley CD, Curtis JR, Baddley JW, Mycobacterial diseases and antitumour necrosis factor therapy in USA. Ann Rheum Dis. 2013;72:37–42. DOIPubMedGoogle Scholar
- Kendall BA, Winthrop KL. Update on the epidemiology of pulmonary nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013;34:87–94. DOIPubMedGoogle Scholar
- Dougados M, Soubrier M, Antunez A, Balint P, Balsa A, Buch MH, Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann Rheum Dis. 2014;73:62–8. DOIPubMedGoogle Scholar
- Lindhardsen J, Ahlehoff O, Gislason GH, Madsen OR, Olesen JB, Torp-Pedersen C, The risk of myocardial infarction in rheumatoid arthritis and diabetes mellitus: a Danish nationwide cohort study. Ann Rheum Dis. 2011;70:929–34 . DOIPubMedGoogle Scholar
- Marais BJ, Lonnroth K, Lawn SD, Migliori GB, Mwaba P, Glaziou P, Tuberculosis comorbidity with communicable and non-communicable diseases: integrating health services and control efforts. Lancet Infect Dis. 2013;13:436–48. DOIPubMedGoogle Scholar
- Lee CH, Lee MC, Lin HH, Shu CC, Wang JY, Lee LN, Pulmonary tuberculosis and delay in anti-tuberculous treatment are important risk factors for chronic obstructive pulmonary disease. PLoS ONE. 2012;7:e37978. DOIPubMedGoogle Scholar
- Lee CH, Lee MC, Shu CC, Lim CS, Wang JY, Lee LN, Risk factors for pulmonary tuberculosis in patients with chronic obstructive airway disease in Taiwan: a nationwide cohort study. BMC Infect Dis. 2013;13:194. DOIPubMedGoogle Scholar
- Yeh JJ, Wang YC, Lin CL, Chou CY, Yeh TC, Wu BT, Nontuberculous mycobacterial infection is associated with increased respiratory failure: a nationwide cohort study. PLoS ONE. 2014;9:e99260. DOIPubMedGoogle Scholar
- The National Health Insurance Statistics. Statistics & surveys [cited 2014 Nov 15]. http://www.nhi.gov.tw/English/webdata/webdata.aspx?menu=11&menu_id=296&webdata_id=1942&WD_ID=296
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. DOIPubMedGoogle Scholar
- Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9. DOIPubMedGoogle Scholar
- Arkema EV, Jonsson J, Baecklund E, Bruchfeld J, Feltelius N, Askling J; ARTIS Study Group. Are patients with rheumatoid arthritis still at an increased risk of tuberculosis and what is the role of biological treatments? Ann Rheum Dis. 2015;74:1212–7 . DOIPubMedGoogle Scholar
- Simons S, van Ingen J, Hsueh PR, Van Hung N, Dekhuijzen PN, Boeree MJ, Nontuberculous mycobacteria in respiratory tract infections, eastern Asia. Emerg Infect Dis. 2011;17:343–9. DOIPubMedGoogle Scholar
- Morimoto K, Iwai K, Uchimura K, Okumura M, Yoshiyama T, Yoshimori K, A steady increase in nontuberculous mycobacteriosis mortality and estimated prevalence in Japan. Ann Am Thorac Soc. 2014;11:1–8.
- Brode SK, Jamieson FB, Ng R, Campitelli MA, Kwong JC, Paterson JM, Risk of mycobacterial infections associated with rheumatoid arthritis in Ontario, Canada. Chest. 2014;146:563–72. DOIPubMedGoogle Scholar
- Ringshausen FC, Apel RM, Bange FC, de Roux A, Pletz MW, Rademacher J, Burden and trends of hospitalisations associated with pulmonary non-tuberculous mycobacterial infections in Germany, 2005–2011. BMC Infect Dis. 2013;13:231. DOIPubMedGoogle Scholar
- Billinger ME, Olivier KN, Viboud C, de Oca RM, Steiner C, Holland SM, Nontuberculous mycobacteria–associated lung disease in hospitalized persons, United States, 1998–2005. Emerg Infect Dis. 2009;15:1562–9. DOIPubMedGoogle Scholar
- Winthrop KL, Chang E, Yamashita S, Iademarco MF, LoBue PA. Nontuberculous mycobacteria infections and anti–tumor necrosis factor-α therapy. Emerg Infect Dis. 2009;15:1556–61. DOIPubMedGoogle Scholar
- Winthrop KL, Iseman M. Bedfellows: mycobacteria and rheumatoid arthritis in the era of biologic therapy. Nat Rev Rheumatol. 2013;9:524–31.
- Mirsaeidi M, Machado RF, Garcia JG, Schraufnagel DE. Nontuberculous mycobacterial disease mortality in the United States, 1999–2010: a population-based comparative study. PLoS ONE. 2014;9:e91879. DOIPubMedGoogle Scholar
- Symmons DP, Bankhead CR, Harrison BJ, Brennan P, Barrett EM, Scott DG, Blood transfusion, smoking, and obesity as risk factors for the development of rheumatoid arthritis: results from a primary care–based incident case–control study in Norfolk, England. Arthritis Rheum. 1997;40:1955–61. DOIPubMedGoogle Scholar
Figures
Tables
Cite This Article1Deceased.
Table of Contents – Volume 21, Number 8—August 2015
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Der-Yuan Chen, Division of Allergy, Immunology and Rheumatology, Department of Medical Education, Taichung Veterans General Hospital, No.1650, Sec.4, Taiwan Blvd, Xitun District, Taichung, Taiwan
Top