Volume 22, Number 9—September 2016
Research
Feasibility of Using Convalescent Plasma Immunotherapy for MERS-CoV Infection, Saudi Arabia
Figure 1
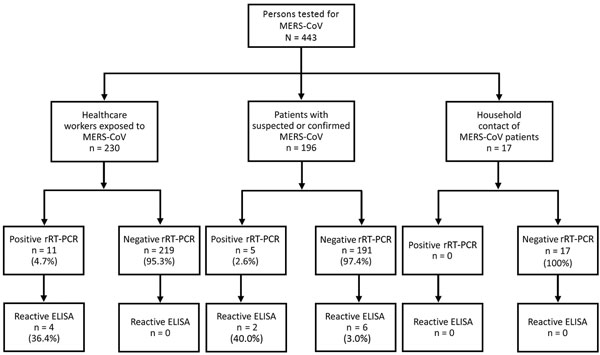
Figure 1. Antibody test results for 443 persons in a study determining the feasibility of using convalescent plasma immunotherapy for Middle East respiratory coronavirus (MERS-CoV) infection, Saudi Arabia. rRT-PCR, real-time reverse transcription PCR.
1This author is a member of ISARIC (the International Severe Acute Respiratory and Emerging Infection Consortium).
Page created: August 16, 2016
Page updated: August 16, 2016
Page reviewed: August 16, 2016
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.