Volume 23, Number 11—November 2017
Dispatch
Lineage-Specific Real-Time RT-PCR for Yellow Fever Virus Outbreak Surveillance, Brazil
Figure 2
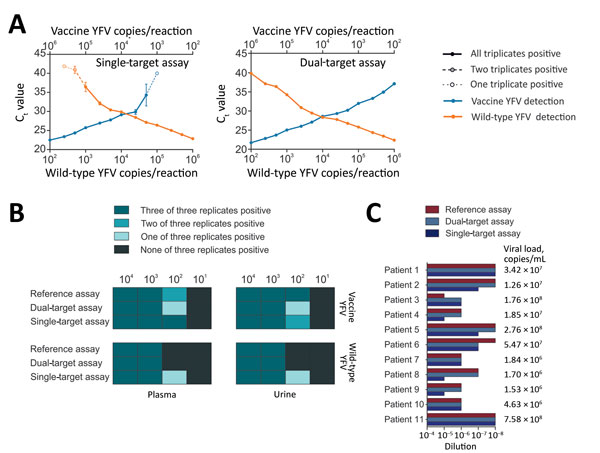
Figure 2. Validation of new real-time RT-PCRs for differentiation between vaccine and wild-type YFV. A) Effects of target competition on YFV real-time RT-PCRs. Mean cycle threshold (Ct) values are plotted against IVT concentrations. Triplicates were tested for each datum point. B) Validation of the assays with clinical matrices. Spiked viruses were vaccine strain 17D and the American genotype 2 wild-type strain BOL88/1999. RNA purification was performed using the MagNA Pure 96 Viral NA Small Volume Kit (Roche, Basel, Switzerland) according to the manufacturer’s instructions. C) Clinical validation. Clinical specimens (serum, liver, whole blood, and plasma) from 11 YFV-infected patients were tested. RNA was extracted using the MagMAX Pathogen RNA/DNA Kit (Thermo Fisher, São Paulo, Brazil) and serial dilutions of the RNA were tested using the new assays and a YFV reference assay (12). Viral loads were determined for clinical specimens using a commercially available quantitative real-time RT-PCR (Bio Gene Research Yellow Fever PCR kit; Bioclin, Minas Gerais, Brazil), following the manufacturer´s instructions. Standard curves and sample copies per millileter were calculated using an in-house IVT standard. IVT, in vitro transcript; RT-PCR, reverse transcription PCR; YFV, yellow fever virus.
References
- Gardner CL, Ryman KD. Yellow fever: a reemerging threat. Clin Lab Med. 2010;30:237–60. DOIPubMedGoogle Scholar
- Beasley DW, McAuley AJ, Bente DA. Yellow fever virus: genetic and phenotypic diversity and implications for detection, prevention and therapy. Antiviral Res. 2015;115:48–70. DOIPubMedGoogle Scholar
- Bryant JE, Holmes EC, Barrett AD. Out of Africa: a molecular perspective on the introduction of yellow fever virus into the Americas. [<jrn>]. PLoS Pathog. 2007;3:e75. DOIPubMedGoogle Scholar
- Ministério da Saúde Brazil. Informe especial febre amarela no Brasil Nº 01/2017: Ministério da Saúde 2017 [cited 2017 Sep 1]. http://portalarquivos.saude.gov.br/images/pdf/2017/marco/18/Informe-especial-COES-FA.pdf
- Bonaldo MC, Gómez MM, Dos Santos AA, Abreu FVS, Ferreira-de-Brito A, Miranda RM, et al. Genome analysis of yellow fever virus of the ongoing outbreak in Brazil reveals polymorphisms. Mem Inst Oswaldo Cruz. 2017;112:447–51. DOIPubMedGoogle Scholar
- World Health Organization. WHO dispatched 3.5 million doses of yellow fever vaccine for outbreak response in Brazil. 2017 30 March 2017 [cited 2017 Aug 30]. http://www.who.int/csr/disease/yellowfev/vaccination-in-Brazil/en/
- de Melo AB, da Silva MP, Magalhães MC, Gonzales Gil LH, Freese de Carvalho EM, Braga-Neto UM, et al. Description of a prospective 17DD yellow fever vaccine cohort in Recife, Brazil. Am J Trop Med Hyg. 2011;85:739–47. DOIPubMedGoogle Scholar
- Thomas RE. Yellow fever vaccine-associated viscerotropic disease: current perspectives. Drug Des Devel Ther. 2016;10:3345–53. DOIPubMedGoogle Scholar
- Boyd AT, Dombaxe D, Moreira R, Oliveira MS, Manuel E, Colorado CN, et al. Notes from the field: investigation of patients testing positive for yellow fever viral RNA after vaccination during a mass yellow fever vaccination campaign—Angola, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:282–3. DOIPubMedGoogle Scholar
- Simmonds P. SSE: a nucleotide and amino acid sequence analysis platform. BMC Res Notes. 2012;5:50–9. DOIPubMedGoogle Scholar
- Corman VM, Rasche A, Baronti C, Aldabbagh S, Cadar D, Reusken CB, et al. Assay optimization for molecular detection of Zika virus. Bull World Health Organ. 2016;94:880–92. DOIPubMedGoogle Scholar
- Domingo C, Patel P, Yillah J, Weidmann M, Méndez JA, Nakouné ER, et al. Advanced yellow fever virus genome detection in point-of-care facilities and reference laboratories. J Clin Microbiol. 2012;50:4054–60. DOIPubMedGoogle Scholar
- World Health Organization. Dengue vaccine: WHO position paper – July 2016. Wkly Epidemiol Rec. 2016;91:349–64.PubMedGoogle Scholar
- Hegde NR, Gore MM. Japanese encephalitis vaccines: Immunogenicity, protective efficacy, effectiveness, and impact on the burden of disease. Hum Vaccin Immunother. 2017;13:1–18. DOIPubMedGoogle Scholar
- Dayan GH, Bevilacqua J, Coleman D, Buldo A, Risi G. Phase II, dose ranging study of the safety and immunogenicity of single dose West Nile vaccine in healthy adults ≥ 50 years of age. Vaccine. 2012;30:6656–64. DOIPubMedGoogle Scholar
1These authors contributed equally to this article.