Volume 23, Number 2—February 2017
Dispatch
Neisseria meningitidis ST11 Complex Isolates Associated with Nongonococcal Urethritis, Indiana, USA, 2015–2016
Cite This Article
Citation for Media
Abstract
At a clinic in Indianapolis, Indiana, USA, we observed an increase in Neisseria gonorrhoeae–negative men with suspected gonococcal urethritis who had urethral cultures positive for N. meningitidis. We describe genomes of 2 of these N. meningitidis sequence type 11 complex urethritis isolates. Clinical evidence suggests these isolates may represent an emerging urethrotropic clade.
Neisseria meningitidis and N. gonorrhoeae are exclusive human pathogens. N. meningitidis is a leading cause of sepsis and meningitis, whereas N. gonorrhoeae (gonococcus) traditionally causes gonorrhea, a sexually transmitted infection involving the genitals, rectum, and throat. These species usually occupy distinct niches but may cause reciprocal diseases when N. meningitidis colonizes the anogenital tract or gonococcus colonizes oropharyngeal mucous membranes (1,2).
Sporadic cases of meningococcal urethritis have been reported since the 1930s (3). It was recognized by the 1970s that urethral N. meningitidis infections could be spread by oral sex and were more common in men who have sex with men (4). Most N. meningitidis urethritis cases identified before 1993 were caused by strains from serogroups A and B (5), but outbreaks involving other serogroups and nontypeable strains have been reported (6–10). These cases have typically presented with purulent urethritis or proctitis with gram-negative intracellular diplococci (GNID) identified by Gram stain of urethral exudates. The current prevalence of meningococcal urethritis is unknown in the United States but was thought to be low (4). However, a recent spike in heterosexual N. meningitidis urethritis cases beginning in 2013 in Ohio and Michigan, reported by Bazan et al. in association with the Gonococcal Isolate Surveillance Project, was linked to a nongroupable clonal N. meningitidis strain (sequence type 11 [ST11], clonal complex [CC] ET-37) (11). Whether N. meningitidis ST11 strains are common causes of nongonococcal urethritis or if these strains have adaptations that enhance their virulence for the urethra is unknown because genomes of urethral N. meningitidis isolates have not been previously reported.
Beginning in early 2015, we observed that 4 (6%) of 59 men we enrolled in gonococcus treatment trials in our clinic in Indianapolis, Indiana, USA, and who had urethral specimens that were positive on Gram stain tested negative for gonococcus by specific nucleic acid amplification tests (NAATs). Sugar fermentation reaction profiles of the isolates from all 4 of these men suggested that they were infected with N. meningitidis. We describe the recent epidemiology of these suspected N. meningitidis urethritis cases and the genomes of 2 of these N. meningitidis isolates.
We enrolled 59 men (age range 20–61 years, median age 31 years) in 3 gonoccocal treatment trials from April 2014 through February 2016 at the Bell Flower Clinic, a public sexually transmitted infections clinic, in Indianapolis, Marion County, Indiana. All of these men had purulent urethral discharge with >10 leukocytes and GNID on Gram stains of urethral exudate. However, 4 men tested negative for gonococcus with specific NAATs (APTIMA Combo 2, Gen-Probe Inc., San Diego, CA, USA; or COBAS 4800, Roche Diagnostics, Indianapolis, IN, USA). All 4 men reported recent vaginal and oral sexual exposures. Urethral cultures yielded growth with colony morphology, oxidase, and Gram stain results consistent with gonococcus, but the isolates fermented glucose and maltose but not lactose, consistent with N. meningitidis. A pharyngeal swab from 1 of these men also grew N. meningitidis, whereas rectal swab specimens from all 4 were culture negative. All 4 cases responded to investigational gonococcal antibiotic regimens and were culture plus NAAT negative by test of cure assessed at day 7 posttreatment. Two of these isolates (NM1 and NM2), which were susceptible to ampicillin, ceftriaxone, chloramphenicol, levofloxacin and meropenem, were subcultured and confirmed to be N. meningitidis by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry.
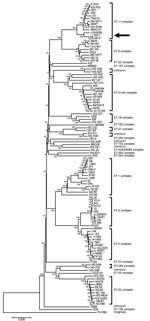
Whole-genome sequencing of NM1 and NM2 (Technical Appendix) revealed that these 2 isolates closely resembled strains of ST11 and CC11, with fine type PorA VR 1.5–1, PorA2 10–8; FetA3–6 ST11 (CC11). Phylogenetic analysis indicated that NM1 and NM2 likely share a common ancestor with NM L93/4286, a serogroup C invasive strain from the United Kingdom (12). A rooted tree constructed by the neighbor-joining method indicated that NM1 and NM2 are closely related to FAM18 (Figure) (13). Gene-to-gene comparison with FAM18 loci revealed that 29 out of 1,975 loci were missing in >1 of the urethral isolates, although 24 of these were near the end of a contig in either NM1 or NM2. Of the remaining loci, 1,075 (56%) were identical in all three strains, and 1,848 (96%) were identical in NM1 and NM2.
The capsule locus encompasses ≈24 kb in most NM isolates and contains genes that mediate capsule synthesis, transport, assembly and translocation, as well as LPS synthesis. NM1 and NM2 lack cssC, cssB, and cssA (former designation siaC, siaB, and siaC [14]), whereas these are intact in NM FAM18 and MC58 (Technical Appendix). Our analysis indicated that these genes are highly conserved in diverse isolates from N. meningitidis serogroups B, C, W, and Y, but are also absent in gonococcal strain FA1090.
To estimate the prevalence of presumptive NM urethritis at BFC, we retrospectively examined 107 cases from men seen between 2013 and 2016 who had Gram stain results positive for WBCs and GNID but who had negative GC NAATs. Rates of negative GC NAATs among men with positive Gram stains were 12/436 (2.8%) in 2013, 8/552 (1.4%) in 2014, 37/533 (6.9%) in 2015, and 50/510 (9.8%) in the first 3 quarters of 2016, indicating a significant increase in these cases during the study interval by both a χ2 test (p<0.0001) comparing the 4 years and the Mantel-Haenszel test for a trend over time (p = 0.005). Analysis of cases before 2013 was not possible because of the lack of discrimination in the NAAT used at the clinic.
Similar to the case patients recently reported from Ohio and Michigan (11), our 37 case-patients in 2015 had an average age of 32 (range 20–61) years and were symptomatic with either discharge (n = 35) or dysuria (n = 2) and predominantly heterosexual (n = 34) (Table). Most received oral sex (n = 35) and were HIV negative (n = 32/32 tested).
Together, our results and those of Bazan (11) suggest that N. meningitidis ST11 could be a notable emerging cause of nongonococcal urethritis; more extensive sequencing and comparisons of recent N. meningitidis ST11 isolates from around the United States are underway. However, our results support the observation that both the rates and geographic distribution of N. meningitidis–associated urethritis cases in the United States are increasing. Sequencing additional isolates should clarify whether these isolates correspond to an emerging urethrotropic clade of N. meningitidis. Retrospective comparisons of NAAT-negative, GNID-positive urethritis case rates might also help discern whether these cases have been increasing elsewhere. As fewer clinics perform routine Gram staining, a serious concern is that infections with urethral discharge, coupled with a negative gonococcus-specific NAAT, could be misdiagnosed as a Chlamydia or Trichomonas infection. We speculate that an increased number of N. meningitidis cases may occur if we limit our clinical diagnoses solely on the results of current diagnostic methods, thereby causing asymptomatic cases to go untreated. Therefore, continued investigation into diagnostic methods targeting urethral specific N. meningitidis isolates is pressing, to control the transmission of sexually transmitted N. meningitidis. Finally, we note that because the meningococcal ctrA gene is highly conserved in NM1 and NM2, they should be able to be differentiated from gonococcus by using meningococcal ctrA reverse transcription PCR assay(15).
Dr. Toh is a postdoctoral fellow at the Indiana University School of Medicine in Indianapolis, Indiana. Her research interests include pathogen discovery using cultivation-independent metagenomic sequencing and comparative genomic approaches and molecular typing of sexually transmitted bacterial pathogens.
Acknowledgments
We thank Rosemary Batteiger for assistance with mass spectrometry. We also thank the Bell Flower Clinic staff for the enrollment of patients into the gonorrhea study and for their meticulous documentation of clinical characteristics of patients. In addition, we are grateful to Stan Spinola for his advice and review of this manuscript.
This work was supported in part by National Institutes of Health grant no. AI116706-01A1 (to D.E.N. and B.E.B.).
References
- Feldman HA. Meningococcus and gonococcus: never the Twain—well, hardly ever. N Engl J Med. 1971;285:518–20. DOIPubMedGoogle Scholar
- Carpenter CM, Charles R. Isolation of meningococcus from the genitourinary tract of seven patients. Am J Public Health Nations Health. 1942;32:640–3. DOIPubMedGoogle Scholar
- Murray E. Meningococcus infections of the male urogenital tract and the liability to confusion with gonococcus infection. Urol Cutaneous Rev. 1939;43:739–41.
- Janda WM, Bohnoff M, Morello JA, Lerner SA. Prevalence and site-pathogen studies of Neisseria meningitidis and N gonorrhoeae in homosexual men. JAMA. 1980;244:2060–4. DOIPubMedGoogle Scholar
- Nebreda T, Campos A, Merino FJ. Urethritis caused by Neisseria meningitidis serogroup C. Clin Microbiol Infect. 1999;5:57–60. DOIPubMedGoogle Scholar
- Rodriguez CN, Rodriguez-Morales AJ, Garcia A, Pastran B, Rios A, Calvo A, et al. Quinolone and azithromycin-resistant Neisseria meningitidis serogroup C causing urethritis in a heterosexual man. Int J STD AIDS. 2005;16:649–50. DOIPubMedGoogle Scholar
- Gregory JE, Crook R, Keeler G. Urethritis attributable to Neisseria meningitidis, group X: a case report. J Natl Med Assoc. 1979;71:845–6.PubMedGoogle Scholar
- Hayakawa K, Itoda I, Shimuta K, Takahashi H, Ohnishi M. Urethritis caused by novel Neisseria meningitidis serogroup W in man who has sex with men, Japan. Emerg Infect Dis. 2014;20:1585–7. DOIPubMedGoogle Scholar
- Hagman M, Forslin L, Moi H, Danielsson D. Neisseria meningitidis in specimens from urogenital sites. Is increased awareness necessary? Sex Transm Dis. 1991;18:228–32. DOIPubMedGoogle Scholar
- Shanmugaratnam K, Pattman RS. Acute urethritis due to Neisseria meningitidis. Genitourin Med. 1989;65:401–2.PubMedGoogle Scholar
- Bazan JA, Peterson AS, Kirkcaldy RD, Briere EC, Maierhofer C, Turner AN, et al. Notes from the field: Increase in Neisseria meningitidis–associated urethritis among men at two sentinel clinics—Columbus, Ohio, and Oakland County, Michigan, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:550–2. DOIPubMedGoogle Scholar
- Bratcher HB, Corton C, Jolley KA, Parkhill J, Maiden MC. A gene-by-gene population genomics platform: de novo assembly, annotation and genealogical analysis of 108 representative Neisseria meningitidis genomes. BMC Genomics. 2014;15:1138. DOIPubMedGoogle Scholar
- Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–4. DOIPubMedGoogle Scholar
- Harrison OB, Claus H, Jiang Y, Bennett JS, Bratcher HB, Jolley KA, et al. Description and nomenclature of Neisseria meningitidis capsule locus. Emerg Infect Dis. 2013;19:566–73. DOIPubMedGoogle Scholar
- Corless CE, Guiver M, Borrow R, Edwards-Jones V, Fox AJ, Kaczmarski EB. Simultaneous detection of Neisseria meningitidis, Haemophilus influenzae, and Streptococcus pneumoniae in suspected cases of meningitis and septicemia using real-time PCR. J Clin Microbiol. 2001;39:1553–8. DOIPubMedGoogle Scholar
Figure
Table
Cite This Article1These authors contributed equally to this article.
Table of Contents – Volume 23, Number 2—February 2017
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Address for corrrespondence: David E. Nelson, Indiana University School of Medicine, Department of Microbiology & Immunology, 635 Barnhill Dr, MS420, Indianapolis, IN 46202, USA
Top