Volume 23, Number 4—April 2017
Research Letter
Bartonella-Associated Transverse Myelitis
Abstract
Each year in the United States, 500 patients are hospitalized for cat-scratch disease, caused by Bartonella henselae infection. We report a case of rare but serious neurologic B. henselae infection. When typical features of cat-scratch disease occur with neurologic findings, Bartonella infection should be suspected and diagnostic testing should be performed.
In a recent epidemiologic study, Nelson et al. (1) estimated that each year in the United States, 500 patients are hospitalized for cat-scratch disease (CSD), caused by Bartonella henselae infection. Typical disease presentation includes enlarged lymph nodes proximal to the site of organism inoculation. B. henselae can disseminate and infect various organs, including the central nervous system (CNS). For patients with neurologic involvement, laboratory diagnosis can be challenging.
In 2015, a previously healthy 46-year-old woman was referred to Bern University Hospital, Bern, Switzerland, for suspected acute ischemic stroke; she was experiencing dysarthria, aphasia, dysphagia, paresthesia, and weakness in both legs. She reported no travel history or recent vaccination but reported having had contact with her neighbor’s cat.
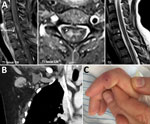
Physical examination revealed blood pressure 130/80 mm Hg and heart rate 100 beats/min. Also noted were flaccid paralysis of the lower extremities (manual muscle testing score 4, dorsal and plantar flexion of the foot; manual muscle testing score 2, flexion and extension of the thighs), dysarthria, peripheral facial paralysis, and gaze-evoked nystagmus. Serum leukocyte count was 7.2 × 109 cells/L (without a left shift of neutrophils), and serum C-reactive protein level was 8 mg/L (reference <5 mg/L). Magnetic resonance images of the brain showed no abnormalities, but those of the spinal cord showed longitudinal lesions consistent with transverse myelitis (Figure). Analyses of cerebrospinal fluid (CSF) revealed a cell count of 167/μL (98% mononuclear cells) and elevated levels of protein (1.1 g/L) and lactate (2.4 mmol/L). The CSF/serum ratio of albumin indicated a blood–CSF barrier dysfunction. Thus, a diagnosis of meningoencephalitis and acute transverse myelitis was made.
Initial treatment consisted of corticosteroids and empirically prescribed antiinfective therapy with acyclovir, amoxicillin, and ceftriaxone. After 5 days of incubation, CSF samples showed no microorganism growth; however, specific culture techniques for Bartonella spp. were not used because lumbar puncture had been performed before CSD was suspected. PCR results were negative for herpes simplex virus 1/2, varicella zoster virus, cytomegalovirus, Epstein-Barr virus, and enterovirus. Serologic test results were negative for Borrelia burgdorferi, Treponema pallidum, Mycoplasma pneumoniae, tickborne encephalitis virus, Toxoplasma gondii, and HIV, as were results for Cryptococcus neoformans serum antigen testing. Results of a multiplex PCR for respiratory viruses and M. pneumoniae in a nasopharyngeal swab sample were negative. Therefore, empirical treatment with antiviral and antibiotic agents was stopped. Because no infectious etiology was found, the differential diagnosis included vasculitis and neoplastic and paraneoplastic disorders. The clinical findings and high CSF cell count argued against multiple sclerosis. Test results were negative for autoantibodies (antinuclear antibodies; c and p antineutrophil cytoplasmic antibodies; double-stranded DNA antibodies; and antiphospholipid, onconeural, and neuromyelitis optica [antiaquaporin 4] autoantibodies).
Computed tomography of the chest and abdomen showed no evidence of neoplasia but did show enlarged right-sided axillary lymph nodes (Figure). This result, together with a visible scratch on the patient’s right index finger (Figure) and a history of contact with the neighbor’s cat, was highly suggestive of CSD. The PCR result for B. henselae (Technical Appendix) in a biopsy sample from the right index finger was positive, as was the result of an indirect immunofluorescence assay for B. henselae IgG (titer 1:512). After being asked, the patient reported having been scratched by the cat 6 weeks earlier and having felt enlarged axillary lymph nodes 4 weeks before admission. The PCR result for B. henselae in CSF was negative. Intrathecal antibody production was not elevated, and the specific CSF/serum ratio for IgG against Bartonella was negative, although the laboratory method for the latter is not standardized. CSD-associated transverse myelitis was postulated. Doxycycline was given for 3 weeks and continued with tapering doses of corticosteroids. The patient improved, but at follow-up examination 6 months after discharge, she reported residual neurologic symptoms, including fatigue, chronic headache, radicular neuropathic pain, and slight gait unsteadiness. B. henselae IgG titer was 1:64.
For this patient, the classic features of CSD were present, and laboratory diagnosis of CSD was made by 2 methods. Nonetheless, we detected neither Bartonella antigens in CSF via PCR nor significant intrathecal production of antibodies against Bartonella. Thus, we cannot rule out coincidental CSD and idiopathic inflammatory myelitis. However, in the latter disease, the CSF cell count is typically markedly lower and the myelitis less extensive than was observed for this patient. Moreover, the consistent time course of the disease, lack of an alternative diagnosis, and similarity to other clinical courses suggest CSD-associated transverse myelitis (2). In previously published cases of patients with CNS manifestations and CSD, laboratory diagnosis was not made from CSF samples (2–6). Therefore, it is uncertain whether the pathogenesis of myelitis is the result of direct invasion of the spinal cord by B. henselae or an immune-mediated postinfectious process (2).
Our report and others (2–6) demonstrate that diagnosis of B. henselae CNS disease is currently based on neurologic symptoms and findings after a cat scratch, not on laboratory diagnosis of CSF or CNS biopsy samples. Nonetheless, for suspected cases of CSD, laboratory studies from serum or skin lesion samples should be used for confirmation.
Dr. Sendi is an attending physician and lecturer in infectious diseases at Bern University Hospital and the University of Bern, Switzerland. His research interests are group B Streptococcus in nonpregnant adults, infections of the locomotor apparatus, and infectious diseases in neurology.
Acknowledgment
We are indebted to Martin Altwegg for his contribution to the development of Bartonella PCR assays.
References
- Nelson CA, Saha S, Mead PS. Cat-Scratch Disease in the United States, 2005-2013. Emerg Infect Dis. 2016;22:1741–6. DOIPubMedGoogle Scholar
- Baylor P, Garoufi A, Karpathios T, Lutz J, Mogelof J, Moseley D. Transverse myelitis in 2 patients with Bartonella henselae infection (cat scratch disease). Clin Infect Dis. 2007;45:e42–5. DOIPubMedGoogle Scholar
- Marra CM. Neurologic complications of Bartonella henselae infection. Curr Opin Neurol. 1995;8:164–9. DOIPubMedGoogle Scholar
- Carman KB, Yimenicioglu S, Ekici A, Yakut A, Dinleyici EC. Co-existence of acute transverse myelitis and Guillain-Barré syndrome associated with Bartonella henselae infection. Paediatr Int Child Health. 2013;33:190–2. DOIPubMedGoogle Scholar
- Massei F, Gori L, Taddeucci G, Macchia P, Maggiore G. Bartonella henselae infection associated with Guillain-Barre syndrome. Pediatr Infect Dis J. 2006;25:90–1. DOIPubMedGoogle Scholar
- Vermeulen MJ, Rutten GJ, Verhagen I, Peeters MF, van Dijken PJ. Transient paresis associated with cat-scratch disease: case report and literature review of vertebral osteomyelitis caused by Bartonella henselae. Pediatr Infect Dis J. 2006;25:1177–81. DOIPubMedGoogle Scholar
Figure
Cite This ArticleTable of Contents – Volume 23, Number 4—April 2017
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Parham Sendi, Department of Infectious Diseases, Bern University Hospital and Institute for Infectious Diseases, University of Bern, Switzerland
Top