Volume 23, Number 8—August 2017
Dispatch
Serologic Evidence of Powassan Virus Infection in Patients with Suspected Lyme Disease1
Figure
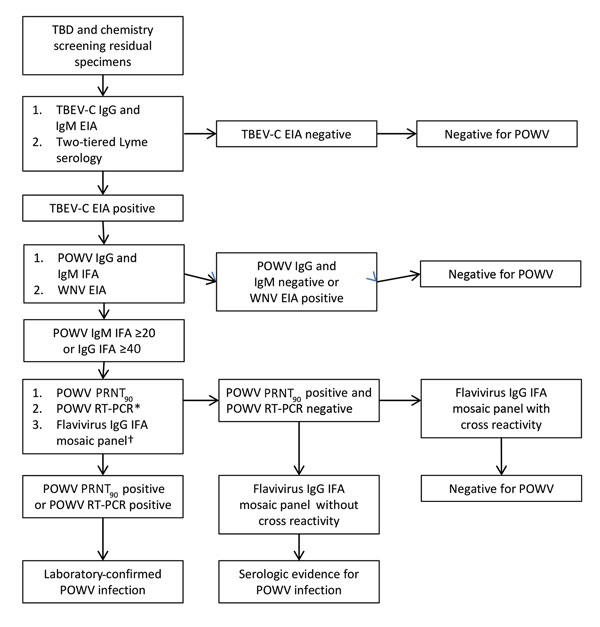
Figure. Flow chart showing series of tests performed on specimens obtained from patients with suspected TBD and patients undergoing routine chemical screening to determine POWV seroreactivity, Wisconsin, July–August 2015. *Performed for TBD samples positive for POWV IgG or IgM and chemical screening samples positive for POWV IgM by IFA assay. †Performed for samples positive for POWV IgG by IFA assay. EIA, enzyme immunoassay; IFA, immunofluorescence antibody assay; POWV, Powassan virus; PRNT90, >90% plaque reduction neutralization test; RT-PCR, reverse transcription PCR; TBD, tickborne disease; TBEV-C, tick-borne encephalitis virus complex; WNV, West Nile virus.
1Preliminary results from this study were presented at IDWeek; October 26–30, 2016; New Orleans, Louisiana, USA.