Volume 24, Number 1—January 2018
Research Letter
Inonotosis in Patient with Hematologic Malignancy
Cite This Article
Citation for Media
Abstract
We report a lung-invasive fungal disease with possible cutaneous needle tract seeding in a patient with a febrile neutropenia caused by the Basidiomycetes mold Inonotus spp. Although rare, Inonotus spp. should be added to the list of microorganisms causing invasive fungal disease in neutropenic patients with hematologic malignancies.
A 33-year-old man in Madrid, Spain, with chronic myeloid leukemia in lymphoid blastic phase underwent allogeneic stem cell transplantation (SCT) from a matched unrelated donor in 2011. Four years later, he had an extramedullary pulmonary relapse, after which he began intensive reinduction chemotherapy. After the second cycle, prolonged severe aplasia developed in the patient. Invasive fungal disease (IFD) was suspected because of the presence of persistent fever despite broad-spectrum antimicrobial drugs and the appearance of a new pulmonary nodule (Figure 1; Technical Appendix) while the patient was receiving prophylactic micafungin (50 mg/d). Serologic fungal biomarkers were negative. A percutaneous pulmonary biopsy sample was taken, and empirical liposomal amphotericin B (3 mg/kg/d) was started (December 2015). Histology showed unspecific inflammatory tissue, and microbiology cultures were negative.
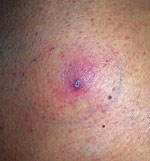
Salvage human leukocyte antigen–haploidentical SCT was performed in January 2016. Fever persisted during conditioning therapy, and a solitary cutaneous millimetric erythematous lesion appeared at the biopsy puncture site (Figure 2). Histopathology of the skin lesion showed dermal infiltration by periodic acid Schiff–positive elements compatible with fungal hyaline hyphae with parallel walls, regular septa, and branched hyphae with occasional bulb-like expansions; angioinvasion; and necrosis. Fungal culture of the specimen was negative, but panfungal PCR and further sequencing (1) revealed the presence of Inonotus spp. Voriconazole was added, and the lesion resolved in days. Neutrophil engraftment was achieved on day 12 post-SCT, with complete donor chimerism.
On day 34, the pulmonary lesion progressed, but we could not prove IFD as the cause of concomitant pleural effusion. Despite intensified antifungal therapy, surgical debridement was required to resolve the empyema. The patient was discharged on oral posaconazole (300 mg/d) that was eventually replaced by micafungin.
A computed tomography scan performed 6 months after the SCT showed persistence of a single mass on the left lung inferior lobe together with a new hepatic nodule. We performed pulmonary segmentectomy, and lung histology showed mycetoma with fungal elements similar to those observed in the previous skin biopsy. The fungal culture yielded a fluffy, white, slow-growing mold. The lack of sporulation did not permit morphologic identification, although panfungal PCR and further sequencing again revealed the presence of Inonotus spp. in the lung tissue sample.
Filamentous Basidiomycetes molds are ubiquitous and able to colonize in patients with chronic pulmonary disease. They cause syndromes comparable to allergic bronchopulmonary or rhinosinusal aspergillosis. However, IFD caused by Basidiomycetes molds are extremely rare (2). It has been hypothesized that IFD could occur in patients with mycetoma if they become immunocompromised. Clinical presentation resembles that of other IFDs, with predominantly pulmonary involvement (Technical Appendix Table 1). Most filamentous Basidiomycetes are susceptible to antifungal drugs except for fluconazole and echinocandins. However, because many isolates do not sporulate, morphologic identification in the clinical microbiology laboratory is difficult without molecular techniques, and antifungal susceptibility testing is impossible to perform (3).
Invasive disease caused by Inonotus spp. (Phellinus tropicalis and P. undulatus) in humans has been described in 1 patient with diabetic nephropathy (3) and 6 patients with chronic granulomatous disease (4–10). Of note, 4 were breakthrough infections in patients receiving prophylactic itraconazole or posaconazole. Local infections had a favorable outcome; however, 1 patient with more extensive involvement had multiple relapses.
The infection in the patient we report mimicked other invasive mold infections in neutropenic patients with hematologic malignancies. However, we observed fungal invasion in the skin after the percutaneous puncture for the pulmonary biopsy, which suggests fungal seeding from the lung source during sample collection.
The presence of fungal elements invading the tissues supported the diagnosis of IFD; nevertheless, we did not initially consider Inonotus spp. to be the causative agent of the IFD in this patient because it rarely causes disease in humans. The clinical significance of the isolation of saprophytic molds in nonsterile clinical samples is difficult to ascertain. However, detection of Inonotus spp. in the lung tissue sample taken months after the skin lesion biopsy led us to reassess its potential role as an etiologic agent. In addition, the patient could have acquired the lung infection after inhalation of spores, and selective pressure of previous antimicrobial drugs could have triggered the breakthrough invasive Inonotus spp. infection. Antifungal therapy was selected without specific recommendations and without antifungal susceptibility testing (because of the poor sporulation of the isolate). Immunosuppression was more profound and prolonged than in other cases of IFD caused by Inonotus spp. Both surgery and antifungal therapy were required, and immunologic recovery, along with a subacute course, were probably essential for the favorable outcome of this patient.
In conclusion, Inonotus spp. should be added to the list of potential causal agents of IFD in neutropenic hematological patients. Systematic use of panfungal PCR targeting the internal transcribed tracer regions coupled with sequencing in patients at a high risk for IFD may be helpful for diagnosing rare invasive fungal infections.
Dr. Fernández-Cruz is an internist and a consultant for hemato-oncological patients in the Clinical Microbiology and Infectious Diseases Department in Hospital Gregorio Marañón in Madrid. Dr. Kwon is a hematology specialist at the Stem Cell Transplant Unit in Hospital Gregorio Marañón in Madrid.
Acknowledgments
We thank Thomas O’Boyle for his help in the preparation of the manuscript and the members of the COMIC (Collaboration in Mycology) study group for their contribution to the work: Almudena Burillo, Amaya Bustinza, Ana Cabrero, Ana Fernández Cruz, Ana Pulido, Antonio Vena, Belén Padilla, Carlos Sánchez, Carmen Rodríguez, Diego Rincón, Eduardo Zarataín, Elena Zamora, Emilio Bouza, Fernando Anaya, Gabriela Rodríguez-Macías, Isabel Frías Soriano, Javier Alarcón, Javier García, Javier Hortal, Jesús Guinea, Jesús Torre, Jorge Gayoso, José Eugenio Guerrero, José María Tellado, José Peral, Lola Vigil, Lorenzo Fernández Quero, Magdalena Salcedo, Mamen Martínez, Manuel Martínez Selles, Manuel Sánchez Luna, María Olmedo, María Sanjurjo, Maricela Valerio, Marina Machado, Marisa Alegre, Marisa Navarro, Marisa Rodríguez, Marta Grande, Mi Kwon, Miguel Martín, Nerea Álava, Paloma Gijón, Patricia Muñoz, Pilar Escribano, Rafael Bañares, Roberto Alonso, Teresa Hernández Sampelayo, and Verónica Parra.
This work was partially supported by grants PI14/00740, PI08/1463, PI11/00708, PI14/01731, MS15/00115, and RD12/0036/0061 and co-financed by Fondo de Investigación Sanitaria (FIS), Instituto de Salud Carlos III; Plan Nacional de I+D+I 2013–2016 and Fondo Europeo de Desarrollo Regional FEDER “Una manera de hacer Europa” support, along with grants from the Fundación LAIR, Asociación Madrileña de Hematología y Hemoterapia (AMHH), Asociación Española Contra el Cáncer (AECC), and Fundación Mutua Madrileña (FMM). FIS supported P.E. (CPI15/00115) and J.G. (CPII15/00006).
The subject of the case report signed an informed consent document for publication.
References
- White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White, TJ, editors. PCR protocols: a guide to methods and applications. New York: Academic Press, 1990. p. 315–22.
- Chowdhary A, Kathuria S, Agarwal K, Meis JF. Recognizing filamentous basidiomycetes as agents of human disease: A review. Med Mycol. 2014;52:782–97. DOIPubMedGoogle Scholar
- Williamson D, Pandey S, Taylor S, Rogers K, Storey L, Marshall MR, et al. A case of infection caused by the basidiomycete Phellinus undulatus. J Med Microbiol. 2011;60:256–8. DOIPubMedGoogle Scholar
- Davis CM, Noroski LM, Dishop MK, Sutton DA, Braverman RM, Paul ME, et al. Basidiomycetous fungal Inonotus tropicalis sacral osteomyelitis in X-linked chronic granulomatous disease. Pediatr Infect Dis J. 2007;26:655–6. DOIPubMedGoogle Scholar
- Ramesh M, Resnick E, Hui Y, Maglione PJ, Mehta H, Kattan J, et al. Phellinus tropicalis abscesses in a patient with chronic granulomatous disease. J Clin Immunol. 2014;34:130–3. DOIPubMedGoogle Scholar
- Nguyen DK, Davis CM, Chinen J, Vallejo JG, Noroski LM. Basidiomycetous Inonotus (Phellinus) tropicalis osteomyelitis in pediatric and adult X-linked chronic granulomatous disease. J Allergy Clin Immunol. 2009;123:S13. DOIGoogle Scholar
- Sutton DA, Thompson EH, Rinaldi MG, Iwen PC, Nakasone KK, Jung HS, et al. Identification and first report of Inonotus (Phellinus) tropicalis as an etiologic agent in a patient with chronic granulomatous disease. J Clin Microbiol. 2005;43:982–7. DOIPubMedGoogle Scholar
- Haidar G, Zerbe CS, Cheng M, Zelazny AM, Holland SM, Sheridan KR. Phellinus species: An emerging cause of refractory fungal infections in patients with X-linked chronic granulomatous disease. Mycoses. 2017;60:155–60. DOIPubMedGoogle Scholar
- Shigemura T, Nakazawa Y, Amano Y, Sudo A, Watanabe M, Kobayashi M, et al. Subcutaneous abscess due to the basidiomycete Phellinus mori in a patient with chronic granulomatous disease. Infection. 2015;43:371–5. DOIPubMedGoogle Scholar
- De Ravin SS, Parta M, Sutton DA, Wickes BL, Thompson EH, Wiederhold NP, et al. Paravertebral mushroom: identification of a novel species of Phellinus as a human pathogen in chronic granulomatous disease. J Clin Microbiol. 2014;52:2726–9. DOIPubMedGoogle Scholar
Figures
Cite This Article1These authors contributed equally to this article.
Table of Contents – Volume 24, Number 1—January 2018
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Ana Fernández-Cruz, Servicio de Microbiología Clínica y Enfermedades Infecciosas, Hospital General Universitario Gregorio Marañón, C/ Dr. Esquerdo, 46, 28007 Madrid, Spain
Top