Volume 24, Number 10—October 2018
Dispatch
Effectiveness of Whole, Inactivated, Low Pathogenicity Influenza A(H7N9) Vaccine against Antigenically Distinct, Highly Pathogenic H7N9 Virus
Figure 2
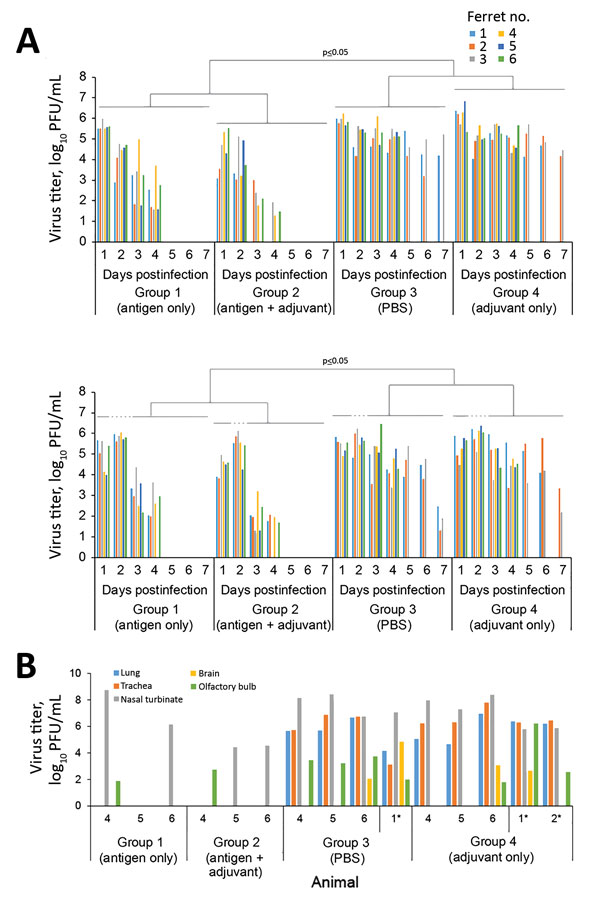
Figure 2. Virus titers in throat and nasal swab specimens and in the organs of vaccinated and nonvaccinated ferrets challenged with highly pathogenic influenza A(H7N9) virus. A) Virus titers in swab samples. Throat and nasal swabs were collected on days 1–7 postchallenge. Virus titers were determined based on plaque assays in MDCK cells. Statistical significance was determined as described in the Technical Appendix. B) Three ferrets from each group were euthanized on day 4 postchallenge for virus titration in the indicated organs. We also assessed virus titers in organs of ferrets that were euthanized because of severe symptoms (*). Virus titers were determined based on plaque assays in MDCK cells. Numbers along baseline indicate animal number. PBS, phosphate-buffered saline.