Volume 24, Number 4—April 2018
Research
Influenza A(H7N9) Virus Antibody Responses in Survivors 1 Year after Infection, China, 2017
Figure 2
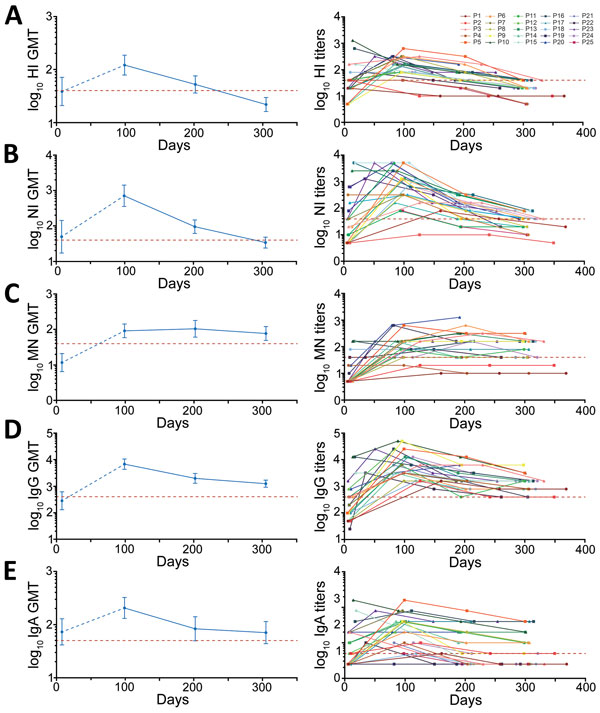
Figure 2. GMTs (left) and individual titers (right) of antibodies to influenza A(H7N9) virus in serum samples collected from survivors, China, 2017: A) HI, B) NI, C) MN, D) IgG, and E) IgA. Red dashed line indicates threshold for seroprotective titer (HI, NI, and MN = 1:40) or limited detection titer (IgG = 1:400; IgA = 1:50). Error bars indicate 95% CIs. GMT, geometric mean titer; HI, hemagglutination inhibition; MN, microneutralization; NI, neuraminidase inhibition; P, patient.
1These authors contributed equally to this article.
Page created: June 22, 2018
Page updated: June 22, 2018
Page reviewed: June 22, 2018
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.