Influenza A(H7N9) Virus Antibody Responses in Survivors 1 Year after Infection, China, 2017
Mai-Juan Ma
1, Cheng Liu
1, Meng-Na Wu, Teng Zhao, Guo-Lin Wang, Yang Yang, Hong-Jing Gu, Peng-Wei Cui, Yuan-Yuan Pang, Ya-Yun Tan, Hui Hang, Bao Lin, Jiang-Chun Qin, Li-Qun Fang

, Wu-Chun Cao

, and Li-Ling Cheng

Author affiliations: Beijing Institute of Microbiology and Epidemiology, Beijing, China (M.-J. Ma, M.-N. Wu, T. Zhao, G.-L. Wang, H.-J. Gu, L.-Q. Fang, W.-C. Cao); University of Florida, Gainesville, Florida, USA (Y. Yang); Suzhou Municipal Center for Disease Control and Prevention, Suzhou, China (C. Liu, P.-W. Cui, Y.-Y. Pang, Y.-Y. Tan, H. Hang, B. Lin, J.-C. Qin, L.-L. Chen)
Main Article
Figure 3
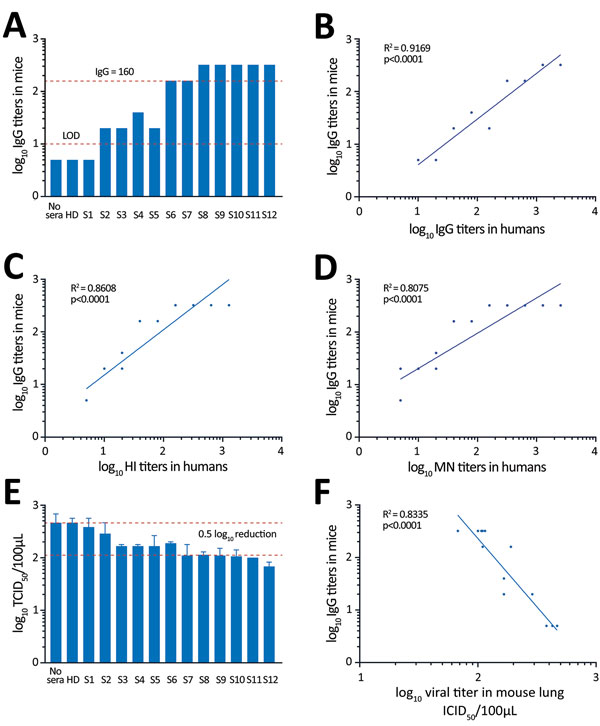
Figure 3. Testing of convalescent-phase serum transfer as potential protection against influenza A(H7N9) virus infection. Mice received 40 μL of patient serum intravenously 12 hours before H7N9 virus infection. A) IgG titers from mouse serum samples collected 1 h before infection. B–D) Relationships between IgG, HI, and MN titers in human serum and IgG titer in mouse recipients of transferred serum. E) Virus titers in homogenized mouse lungs at day 3 after infection (mean ± SE). F) Relationship between IgG titer in mouse serum samples and viral titers in mouse lung samples. HD, healthy donor; HI, hemagglutination inhibition; LOD, limit of detection; MN, microneutralization; S, serum; TCID50, 50% tissue culture infectious dose.
Main Article
Page created: June 22, 2018
Page updated: June 22, 2018
Page reviewed: June 22, 2018
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
