Volume 25, Number 2—February 2019
Dispatch
Lyme Disease Emergence after Invasion of the Blacklegged Tick, Ixodes scapularis, Ontario, Canada, 2010–2016
Cite This Article
Citation for Media
Abstract
Analysis of surveillance data for 2010–2016 in eastern Ontario, Canada, demonstrates the rapid northward spread of Ixodes scapularis ticks and Borrelia burgdorferi, followed by increasing human Lyme disease incidence. Most spread occurred during 2011–2013. Continued monitoring is essential to identify emerging risk areas in this region.
Lyme disease (LD) is the most reported vectorborne disease in North America, where it is caused by Borrelia burgdorferi sensu stricto and principally transmitted by the blacklegged tick (Ixodes scapularis) (1). With northward expansion of I. scapularis tick populations from endemic areas in the United States, LD is rapidly emerging in parts of central and eastern Canada (2–4). Although several studies have mapped blacklegged tick populations across Canada and developed models to predict future spread of ticks and LD risk (2,3), little is known about the extent of human LD in relation to tick vector distributions at a fine geographic scale. We examined spatiotemporal trends in the occurrence and expansion of I. scapularis ticks, B. burgdorferi–infected ticks, and human LD cases over a 7-year period to elucidate the process of LD emergence in eastern Ontario, Canada.
Our study included 3 public health units in eastern Ontario, Canada: Kingston, Frontenac, and Lennox and Addington (KFL); Leeds, Grenville, and Lanark (LGL); and Ottawa. This region spans from the St. Lawrence River in the south to the Ottawa River in the north, and has several major population centers, including Kingston (2016 population 123,798) and Ottawa (2016 population 934,243) (5). The region is largely characterized by mixed deciduous forest and agricultural land use.
We used data from the Integrated Public Health Information System database to identify human LD cases on the basis of provincial case definitions (6). We geocoded cases to their forward sortation area (FSA) (i.e., first 3 digits of the postal code) of residence and extracted data on patient sex, age, episode date (onset of symptoms), and reported history of travel (defined as travel outside the municipality of residence within the previous 2 weeks). Data on ticks collected during 2010–2016 through passive tick surveillance activities in Ontario were obtained from Public Health Ontario (PHO) (7). We aggregated I. scapularis tick records according to the FSA of the submitter (i.e., location of residence of the person who acquired the tick) and excluded records with missing collection date, submitter FSA, or PCR test result and records with reported history of travel. We similarly excluded human LD records with missing patient FSA or with reported travel history. We obtained FSA-level population data for 2011 and FSA boundary files from Statistics Canada (5).
To examine the association between the invasion of I. scapularis ticks and B. burgdorferi and the spread of human LD, we examined associations between FSA-level data on time to first case (in years) and several variables: time to first reported I. scapularis tick, time to first reported B. burgdorferi–infected tick, distance to FSA with highest LD incidence in 2010, and population. We constructed bivariable and multivariable linear regression models with time to first case (in years) as the outcome.
To visualize LD spread during 2010–2016, we plotted the annual FSA-level incidence of human LD and B. burgdorferi prevalence in ticks by using ArcGIS 10.4 (ESRI, https://www.esri.com). We also assessed the annual weighted mean center and distribution of human LD incidence by using ArcGIS 10.4, after spatial projection of the data to preserve distance (8). We applied Kulldorff’s spatial scan statistics (9) by using SaTScan 9.6 (https://www.satscan.org) to assess and compare spatiotemporal patterns in human LD incidence and B. burgdorferi prevalence in ticks at the FSA level (FSA centroids). (For additional methods, see the Appendix).
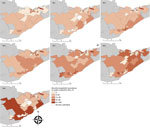
A higher proportion of LD cases occurred in men and in adults 50–69 years of age (Table 1), similar to patterns observed in other regions of North America (10). LD incidence increased over time; 55% of cases occurred during 2015 and 2016 (Table 2). Roughly 70% of cases occurred during June–August, whereas ≈20% occurred during September–December. The number of collected ticks increased annually from 2010 and reached a peak in 2013, with a subsequent decrease because of reductions in passive surveillance activities in KFL and LGL (11); Ottawa received an increasing amount of ticks over time (Table 2). The percentage of ticks testing positive for B. burgdorferi increased annually, from 12% in 2010 to 23% in 2016 (p<0.001). Infection rates were higher among regions of KFL and LGL, although FSAs with high B. burgdorferi prevalence among submitted ticks were observed in parts of Ottawa in more recent years (Figure 1).
Within our study area, the first human LD case was reported an average of 2.2 years after the first reported I. scapularis tick and 1.1 years after the first reported B. burgdorferi–infected tick. Time to first case was significantly associated with time to first reported I. scapularis tick (adjusted r2 = 0.56; p<0.001) and time to first B. burgdorferi–infected tick (adjusted r2 = 0.67; p<0.001) after adjusting for distance to the FSA with highest LD incidence in 2010. The associated lag between each phase of ≈1 year supports the hypothesis that invasion and establishment of tick populations is followed by colonization of B. burgdorferi (12), or it might reflect the arrival of infected ticks with subsequent increase in B. burgdorferi prevalence. However, drawing conclusions on the exact timing of tick and pathogen invasion is difficult because of the nature of passive surveillance data.
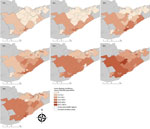
LD incidence was concentrated in southern FSAs in 2010 and 2011 but had spread in a northeasterly direction by 2013 (Figure 2). Overall, a northeast shift of 54 km occurred between mean centers during 2010–2016, with the greatest spread observed in 2011–2013 (Appendix). We detected a spatiotemporal cluster of high rates of B. burgdorferi–infected ticks in the Kingston-Gananoque region bordering the St. Lawrence River, which overlapped with 2 clusters of human LD cases (Appendix Figure 4). The overlapping clusters support the conclusion that increased tick encounter is a determinant of human LD risk. Residence in endemic areas (i.e., where infected ticks have been found) has been consistently recognized as a risk factor for LD infection (13,14).
Although LD incidence in Ottawa had reached ≈7 cases/100,000 population by 2015–2016, the observed incidence rates in KFL and LGL during this period were 4-fold higher (≈30 cases/100,000 population). By comparison, these rates are still far below the ≈110 cases/100,000 population observed in the bordering St. Lawrence County of New York state (15). Given the ongoing emergence process, LD incidence will likely continue to increase in eastern Ontario as I. scapularis tick populations and B. burgdorferi continue to establish and fill in suitable habitats (12). This pattern highlights the importance of fine-scale studies to identify patterns and determinants of LD and other tickborne pathogens in different regions and populations.
Our study was limited by the availability of information on location of tick acquisition and patient exposure location. As such, we aggregated data at the FSA level on the basis of location of patient and tick submitter residence and excluded case-patients and tick submitters with reported travel outside their municipality of residence. Spatiotemporal analysis based on the location of exposure would help to more precisely determine the timing and rate of spread.
Altogether, our findings indicate that LD has emerged in eastern Ontario over a relatively short timescale after the invasion of I. scapularis ticks and B. burgdorferi. Tick surveillance data can serve to identify areas of risk for LD emergence.
Dr. Kulkarni is a medical entomologist and assistant professor in the School of Epidemiology and Public Health at the University of Ottawa. Her research interests include vectorborne diseases with a focus on mosquitoborne and tickborne diseases in Canada and sub-Saharan Africa.
Acknowledgments
The authors would like to thank Dara Spatz Friedman, John Cunningham, and Adam van Dijk for their epidemiologic assistance and data provision. We also thank Ann Stanton-Loucks, Michael Bennitz, and the Ottawa Public Health Innovation Fund for their collaboration and data sharing on tick surveillance activities in Ottawa.
M.A.K. is supported by a grant from the Canadian Institutes for Health Research and by an Early Researcher Award from the Ontario Ministry of Research and Innovation.
Ethics approval for this study was obtained from the Ottawa Public Health Research Ethics Board (no. 226-16) and the University of Ottawa Science and Health Sciences Research Ethics Board (H06-16-22). Access to Integrated Public Health Information System data was granted through data-sharing agreements with participating public health units. All data were aggregated to preserve anonymity.
References
- Bacon RM, Kugeler KJ, Mead PS; Centers for Disease Control and Prevention (CDC). Surveillance for Lyme disease—United States, 1992-2006. MMWR Surveill Summ. 2008;57:1–9.PubMedGoogle Scholar
- Ogden NH, Bouchard C, Kurtenbach K, Margos G, Lindsay LR, Trudel L, et al. Active and passive surveillance and phylogenetic analysis of Borrelia burgdorferi elucidate the process of Lyme disease risk emergence in Canada. Environ Health Perspect. 2010;118:909–14. DOIPubMedGoogle Scholar
- Ogden NH, St-Onge L, Barker IK, Brazeau S, Bigras-Poulin M, Charron DF, et al. Risk maps for range expansion of the Lyme disease vector, Ixodes scapularis, in Canada now and with climate change. Int J Health Geogr. 2008;7:24. DOIPubMedGoogle Scholar
- Ogden NH, Lindsay LR, Morshed M, Sockett PN, Artsob H. The emergence of Lyme disease in Canada. CMAJ. 2009;180:1221–4. DOIPubMedGoogle Scholar
- Statistics Canada. Focus on Geography Series, 2016 census. Statistics Canada catalogue no. 98–404–X2016001. Ottawa (Ontario, Canada): Statistics Canada; 2017.
- Ministry of Health and Long-Term Care. Infectious disease protocol appendix B: provincial case definitions for reportable diseases. Toronto (Ontario, Canada): Ministry of Health and Long-Term Care; 2017.
- Nelder MP, Russell C, Lindsay LR, Dhar B, Patel SN, Johnson S, et al. Population-based passive tick surveillance and detection of expanding foci of blacklegged ticks Ixodes scapularis and the Lyme disease agent Borrelia burgdorferi in Ontario, Canada. PLoS One. 2014;9:e105358. DOIPubMedGoogle Scholar
- Mitchell A. The ESRI guide to GIS analysis. Vol. 2. Redlands (CA, USA): ESRI; 2005.
- Kulldorff M. A spatial scan statistic. Commun Stat Theory Methods. 1997;26:1481–96. DOIGoogle Scholar
- Schwartz AM, Hinckley AF, Mead PS, Hook SA, Kugeler KJ. Surveillance for Lyme disease—United States, 2008–2015. MMWR Surveill Summ. 2017;66:1–12. DOIPubMedGoogle Scholar
- Ontario Agency for Health Protection and Promotion. Technical report: update on Lyme disease prevention and control. Toronto (Ontario, Canada): Ontario Agency for Health Protection and Promotion; 2016.
- Clow KM, Leighton PA, Ogden NH, Lindsay LR, Michel P, Pearl DL, et al. Northward range expansion of Ixodes scapularis evident over a short timescale in Ontario, Canada. Wooten RM, editor. PLoS One. 2017;12:e0189393.
- Cromley EK, Cartter ML, Mrozinski RD, Ertel S-H. Residential setting as a risk factor for Lyme disease in a hyperendemic region. Am J Epidemiol. 1998;147:472–7. DOIPubMedGoogle Scholar
- Finch C, Al-Damluji MS, Krause PJ, Niccolai L, Steeves T, O’Keefe CF, et al. Integrated assessment of behavioral and environmental risk factors for Lyme disease infection on Block Island, Rhode Island. PLoS One. 2014;9:e84758. DOIPubMedGoogle Scholar
- New York State Department of Health. Lyme disease incidence per 100,000. Bureau of Communicable Disease Control Data 2013–2015. New York: New York State Department of Health; 2017.
Figures
Tables
Cite This ArticleOriginal Publication Date: January 07, 2019
Table of Contents – Volume 25, Number 2—February 2019
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Manisha A. Kulkarni, University of Ottawa Faculty of Medicine, School of Epidemiology and Public Health, 600 Peter Morand Crescent, Ottawa, ON K1G 5Z3, Canada
Top