Volume 25, Number 3—March 2019
Research Letter
Biomphalaria pfeifferi Snails and Intestinal Schistosomiasis, Lake Malawi, Africa, 2017–2018
Cite This Article
Citation for Media
Abstract
Two surveys conducted in 2017 and 2018 demonstrated Biomphalaria pfeifferi snails in Lake Malawi in Africa. Epidemiologic examination of 175 local children at 3 primary schools confirmed emergence of intestinal schistosomiasis. These findings highlight autochthonous transmission of Schistosoma mansoni flukes in Lake Malawi and the need to revise international travel advice.
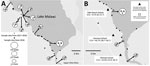
Throughout sub-Saharan Africa, Biomphalaria pfeifferi snails are freshwater intermediate hosts for Schistosoma mansoni blood flukes, which cause intestinal schistosomiasis (1). Geographic distribution of B. pfeifferi snails delineates actual or potentially active zones of S. mansoni fluke transmission (2). Other than a report of a single Biomphalaria shell at Karonga in the far northern portion of Lake Malawi (3), considered to be from a marginal swamp (4), B. pfeifferi snails have not previously been found in Lake Malawi (5). However, in November 2017, during malacologic surveillance for intermediate hosts of schistosomiasis in the Mangochi District, Malawi, along the southernmost tip of Lake Malawi, 2 discrete populations of Biomphalaria snails were unexpectedly encountered in submerged beds of Vallisneria spp. plants (Figure, panel A). DNA sequence analysis of the mitochondrial cytochrome oxidase subunit 1 (cox1) (6) indicated that the cox1 sequences (1,006 bp) of those snails differed from sequences of B. pfeifferi snails from Chiweshe, Zimbabwe (GenBank accession nos. DQ084829 [HCO/LCO region] and DQ084872 [Asmit1/2 region]) by only 3 synonymous single-nucleotide polymorphisms.
In May 2018, to confirm B. pfeifferi colonization within the lake and suspected risk for intestinal schistosomiasis, we undertook a conjoint malacologic and parasitologic survey with ethics approvals from the Liverpool School of Tropical Medicine, UK (application 17-018) and the Ministry of Health and Population, Malawi (application 1805). Reinspection of all prior malacologic sampling locations and another 43 sites found further populations of B. pfeifferi snails (Figure, panel A); large numbers (>50), along with innumerable dead shells, were again found at site 9. All snails were inspected for shedding cercariae, and although cercariae from snails at site 5 were seen, identification by microscopy (×100) was unsuccessful. Supplementary analysis indicated that cox1 sequences from 9 snails from sites 2, 5, 7, 10, and 11 were identical.
We conducted an epidemiologic survey of 175 school children, 5–15 years of age, equal numbers of boys and girls, from 3 primary schools closest to site 9 (Figure, panel B). Mean prevalence of intestinal schistosomiasis, calculated by detection of S. mansoni circulating cathodic antigen (CCA) on urine dipstick testing, was 34.3% (95% CI 27.9–41.3); prevalence rates by school were Samama, 46.7% (95% CI 36.7–56.7); Mchoka, 25.0% (95% CI 15.0–36.7); and Palm Beach, 9.1% (95% CI 0.0–22.7). We requested fecal samples from 60 S. mansoni–positive children and received samples from 46. Duplicate Kato-Katz examinations confirmed S. mansoni ova in 7 children; infection intensities were graded as light (<100 eggs/g feces). All urine samples were inspected for S. haematobium ova by syringe filtration (10 mL); general prevalence was 14.9% (95% CI 9.8–20.1); 52% of these samples were also positive by CCA urine dipstick, indicative of S. mansoni co-infection. To further determine autochthonous transmission of S. mansoni flukes, 2 egg-positive children from Samama and Mchoka took us, on foot, to the shoreline where they regularly swam, which corresponded to snail collection sites 10 and 11 (Figure, panel B). Children who were positive for either S. mansoni CCA or S. haematobium eggs received praziquantel (40 mg/kg).
Colonization of B. pfeifferi snails in Lake Malawi and surrounding water is of concern, especially because active S. mansoni infections were found in local children. This finding highlights emergence of intestinal schistosomiasis, not previously documented here (5,7,8) or detected in this region by the most recent national survey (F. Fleming, Schistosomiasis Control Initiative, Imperial College London; 2017 Dec 20; pers. comm).
Intestinal schistosomiasis has been detected in children ≈150 km away, along the shoreline of the Lower Shire River (9). Finding snails and infected children in Mangochi District suggests recent ecologic and epidemiologic change. In May 2018, the lake was ≈75–80 cm higher than in November 2017, which perhaps favored detection of B. pfeifferi snails in the previously more accessible Vallisneria plant beds. Seasonal dynamics, such as lake level fluctuations, are well known, along with longer duration perturbations of the lake biota, either induced by climate change or mediated by anthropogenic activities. These changes have altered transmission of urogenital schistosomiasis (10); overfishing, particularly of the molluscivorous fish Trematocranus placodon, is changing the distribution of many freshwater snails (5).
Local aquaculture of fish (e.g., Oreochromis spp., called chambo) through use of water pumped inland from the lake has created novel, permanent water bodies colonized by B. pfeifferi snails (e.g., sites 2–7), which may now (re)seed snails into the lake for further establishment. Absence of cox1 genetic diversity in the B. pfeifferi snails we sampled implies a limited number or even a single founder event, but as conditions for autochthonous transmission became favorable, after introduction of S. mansoni flukes, intestinal schistosomiasis in local schoolchildren has emerged. This finding is of substantial public health concern in light of current control efforts, which consist only of annual praziquantel distribution in schools (7,8). We recommend increased surveillance of snails and characterization of schistosomes, along with intensified control interventions to arrest further spread of intestinal schistosomiasis. We also recommend revising and updating health and travel advice given to shoreline community residents and tourists who use the lake.
Mr. Alharbi is a PhD student under the supervision of L.J. and J.R.S. He has specific interests in medical malacology and molecular epidemiology of schistosomiasis in Africa and the Kingdom of Saudi Arabia.
Acknowledgments
We thank Alexandra Shaw and Joanna Fawcett for assistance during the epidemiologic survey in the Mangochi District. We are grateful to the local health and education authorities of Malawi; district teachers; local community health workers Flora Jumbe, Caroline Nthubula, Angelina Mwenyewe, and Witness Mapira; and hosting communities for their enthusiasm and support. We are also indebted to Danie and Hazel Britz for assistance at Palm Beach School, to Paul and Stacey Kennedy for local boat hire, and to Anthony Butterworth and Liz Corbett for their kind hospitality in Blantyre.
M.H.A. was funded by a PhD scholarship from the Ministry of Health, Kingdom of Saudi Arabia, and S.K. by the Commonwealth Scholarship Commission. CCA urine dipsticks were supplied by Rapid Medical Diagnostics (http://www.rapid-diagnostics.com), South Africa (lot no. 171103130).
References
- Brown DS. Freshwater snails of Africa and their medical importance, 2nd ed. London: Taylor & Francis; 1994. p. 321.
- Stensgaard AS, Utzinger J, Vounatsou P, Hürlimann E, Schur N, Saarnak CF, et al. Large-scale determinants of intestinal schistosomiasis and intermediate host snail distribution across Africa: does climate matter? Acta Trop. 2013;128:378–90. DOIPubMedGoogle Scholar
- Smith HE. On a collection of land and freshwater snails transmitted by Mr H.H. Johnston C.B. from British Central Africa. Proc Zoo Soc (London). 1893;632–641.
- Mandahl-Barth G. The freshwater Mollusca of Lake Malawi. Rev Zool Bot Afr. 1972;86:129–60.
- Madsen H, Bloch P, Makaula P, Phiri H, Furu P, Stauffer JR Jr. Schistosomiasis in Lake Malaŵi villages. EcoHealth. 2011;8:163–76. DOIPubMedGoogle Scholar
- Jørgensen A, Kristensen TK, Stothard JR. Phylogeny and biogeography of African Biomphalaria (Gastropoda: Planorbidae), with emphasis on endemic species of the great East African lakes. Zool J Linn Soc. 2007;151:337–49. DOIGoogle Scholar
- Mtethiwa AH, Nkwengulila G, Bakuza J, Sikawa D, Kazembe A. Extent of morbidity associated with schistosomiasis infection in Malawi: a review paper. Infect Dis Poverty. 2015;4:25. DOIPubMedGoogle Scholar
- Makaula P, Sadalaki JR, Muula AS, Kayuni S, Jemu S, Bloch P. Schistosomiasis in Malawi: a systematic review. Parasit Vectors. 2014;7:570. DOIPubMedGoogle Scholar
- Poole H, Terlouw DJ, Naunje A, Mzembe K, Stanton M, Betson M, et al. Schistosomiasis in pre-school-age children and their mothers in Chikhwawa district, Malawi with notes on characterization of schistosomes and snails. Parasit Vectors. 2014;7:153. DOIPubMedGoogle Scholar
- Van Bocxlaer B, Albrecht C, Stauffer JR Jr. Growing population and ecosystem change increase human schistosomiasis around Lake Malaŵi. Trends Parasitol. 2014;30:217–20. DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: January 02, 2019
Table of Contents – Volume 25, Number 3—March 2019
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
J. Russell Stothard, Liverpool School of Tropical Medicine, Parasitology Department, Pembroke Place, Liverpool Merseyside L3 5QA, UK
Top