Volume 31, Number 9—September 2025
Research Letter
Detection of Rat Lungworms in Invasive Mollusks, Georgia, USA, 2024
Cite This Article
Citation for Media
Abstract
The rat lungworm, Angiostrongylus cantonensis, is an invasive, zoonotic parasite that can cause severe disease in humans. We collected A. cantonensis larvae from 2 host species, invasive apple and mystery snails, from bodies of water in Georgia, USA. Recreational water users should avoid ingesting potentially infected hosts, aquatic vegetation, and water.
The rat lungworm, Angiostrongylus cantonensis (Nematoda: Angiostrongylidae), is an invasive human pathogen in many countries, including the United States. This nematode naturally parasitizes rodents (1–3); a variety of gastropod mollusks, typically terrestrial gastropods, act as intermediate hosts. However, aquatic and semiaquatic mollusks, such as invasive apple snails (Pomacea spp.) and mystery snails (Cipangopaludina spp.), have been reported as intermediate hosts (4,5). Freshwater crustaceans, amphibians, reptiles, and flatworms might serve as paratenic hosts (1–4). Infective third-stage level (L3) nematode larvae can also be found on vegetation exposed to infected snails (1). When L3 larvae are ingested by rats, the larvae migrate through vasculature, reaching the central nervous system, and later develop into adults in the pulmonary arteries. In humans, accidental ingestion of rat lungworm can cause severe pathology, including meningitis, or death when L3 larvae migrate to the central nervous system (6).
Rat lungworms are native to Southeast Asia but have spread worldwide (4); the parasite was first reported in the United States in Hawaii in 1960 (2). It was not detected again until 1986 in Louisiana. Recent years have seen a geographic expansion of this parasite: 2013 in Florida, Mississippi, and Texas; 2014 in Alabama and California; 2015 in Oklahoma; 2019 in South Carolina; and 2019–2022 in Georgia (2,4,5,7–9). Despite the broad geographic distribution of rat lungworm, few cases of human angiostrongyliasis have been detected in the United States (8). We collected 2 rat lungworm host species, invasive apple snails (Pomacea maculata) and mystery snails (Cipangopaludina japonica), in bodies of water in Georgia and tested them for A. cantonensis larvae.
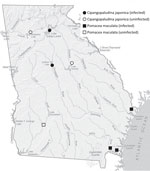
We collected the snails from 8 water bodies in 7 counties during May–October 2024 (Table; Figure). We sampled 430 apple snails (Camden, Chatham, and Dougherty Counties) and 2,562 mystery snails (Cherokee, Greene, Hall, and Jasper Counties) and screened them for nematodes (Appendix, https://wwwnc.cdc.gov/EID/article/31/9/25-0133-App1.pdf). A total of 14 snails (5 mystery snails, 9 apple snails) were infected with rat lungworm. No variation was detected among cox1 sequences from the nematodes. BLAST analysis (https://blast.ncbi.nlm.nih.gov) showed a 100% match to A. cantonensis parasites previously collected in Atlanta (9). Among sites sampled for mystery snails, we detected rat lungworm from Lake Lanier (Hall County; prevalence 18.0/1,000 snails) and the Ocmulgee River (Jasper County; prevalence 6.3/1,000 snails), whereas mystery snails from Lakes Allatoona (Cherokee County) and Oconee (Greene County) were not infected. Apple snails taken from ponds and marshes in Kingsland (prevalence 189.2/1,000 snails) and St. Marys (prevalence 8.5/1,000 snails), both in Camden County, and from Pipemakers Canal (Chatham County; prevalence 4.5/1,000 snails), were infected with rat lungworm, but those from Lake Chehaw (Dougherty County) were not infected (Table). Despite our broad sampling of snails, we detected low overall prevalence of rat lungworm.
Apple snails were first reported in Georgia in 1974 but not reported again until 2005 (10). The early records of the snails were limited to southern Georgia, but in 2013, apple snails were reported in Rockdale County in north central Georgia (10). In the early 2020s, apple snails began to be reported more frequently in the central and northern parts of the state, including in and around the Atlanta metropolitan area (10). Mystery snails are a more recent introduction to Georgia; they were first reported in the state in 2013 from the Atlanta metropolitan area (Clayton and Fulton Counties) (10). Reports of mystery snails throughout the state have become increasingly frequent in recent years (10). We anticipate that future sampling of these snails statewide will show a general trend of increasing prevalence as the invasive snail populations become more established and widespread.
Humans can be infected with rat lungworm by ingesting the molluscan intermediate host or the paratenic host (e.g., crustaceans) or by swallowing infective (L3) larvae, which are found on vegetation (1–3). Apple snails are commonly consumed in some communities in the United States, including Georgia (C.H. Chun, J. Page, M. Rowe, pers. observ.). However, risk of contracting rat lungworm infection is low. Thoroughly cooking the infected snail or paratenic host kills the nematodes and prevents infection; however, accidental exposure through ingesting contaminated vegetation poses a greater human health risk (1). Prior studies have suggested potential human infection through contaminated drinking water (3).
In conclusion, whereas public health decisions related to this parasite should be left to the Centers for Disease Control and Prevention and the Georgia Department of Public Health, we encourage efforts to educate recreational water users to avoid ingesting potentially infected hosts, aquatic vegetation, and water. Long-term management and monitoring of the invasive snail and rodent populations are needed to help minimize the potential spread of rat lungworm and human infection risk in Georgia.
Dr. Achatz is a professor in the Department of Natural Sciences at Middle Georgia State University, Macon, Georgia, USA. His research focuses on parasite taxonomy and systematics as well as disease ecology related to parasitic organisms.
Acknowledgments
We thank Anakela Escobar and the Region 1 fisheries staff (Georgia Department of Natural Resources) for providing mystery snails from Lake Allatoona. We are also grateful to Sarah A. Orlofske (University of Wisconsin – Stephens Point) and Robert A. Newman (University of North Dakota) for their assistance with data analysis.
This work was supported by the University System of Georgia Stem Initiative IV and Center for Middle Georgia Studies.
References
- Niebuhr CN, Jarvi SI, Siers SR. A review of rat lungworm infection and recent data on its definitive hosts in Hawaii. Hum Wildl Interact. 2019;13:238–49.
- Underwood EB, Walker MJ, Darden TL, Kingsley-Smith PR. Frequency of occurrence of the rat lungworm parasite in the invasive island apple snail in South Carolina, USA. J Aquat Anim Health. 2019;31:168–72. DOIPubMedGoogle Scholar
- Jarvi S, Prociv P. Angiostrongylus cantonensis and neuroangiostrongyliasis (rat lungworm disease): 2020. Parasitology. 2021;148:129–32. DOIPubMedGoogle Scholar
- Burns RE, Bicknese EJ, Qvarnstrom Y, DeLeon-Carnes M, Drew CP, Gardiner CH, et al. Cerebral Angiostrongylus cantonensis infection in a captive African pygmy falcon (Polihierax semitorquatus) in southern California. J Vet Diagn Invest. 2014;26:695–8. DOIPubMedGoogle Scholar
- Al Hammoud R, Nayes SL, Murphy JR, Heresi GP, Butler IJ, Pérez N. Angiostrongylus cantonensis meningitis and myelitis, Texas, USA. Emerg Infect Dis. 2017;23:1037–8. DOIPubMedGoogle Scholar
- Gottdenker NL, Nascimento Ramos RA, Hakimi H, McHale B, Rivera S, Miller BM, et al. Angiostrongylus cantonensis infection in brown rats (Rattus norvegicus), Atlanta, Georgia, USA, 2019–2022. Emerg Infect Dis. 2023;29:2167–70. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: August 11, 2025
Table of Contents – Volume 31, Number 9—September 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Tyler J. Achatz, Middle Georgia State University, 100 University Pkwy, Jones Bldg, Rm 372, Macon, GA 31206, USA
Top