Middle East Respiratory Syndrome Coronavirus Infection Dynamics and Antibody Responses among Clinically Diverse Patients, Saudi Arabia
Hail M. Al-Abdely
1, Claire M. Midgley
1
, Abdulrahim M. Alkhamis, Glen R. Abedi, Xiaoyan Lu, Alison M. Binder, Khalid H. Alanazi, Azaibi Tamin, Weam M. Banjar, Sandra Lester, Osman Abdalla, Rebecca M. Dahl, Mutaz Mohammed, Suvang Trivedi, Homoud S. Algarni, Senthilkumar K. Sakthivel, Abdullah Algwizani, Fahad Bafaqeeh, Abdullah Alzahrani, Ali Abraheem Alsharef, Raafat F. Alhakeem, Hani A. Aziz Jokhdar, Sameeh S. Ghazal, Natalie J. Thornburg, Dean D. Erdman, Abdullah M. Assiri, John T. Watson

, and Susan I. Gerber
Author affiliations: Ministry of Health, Riyadh, Saudi Arabia (H.M. Al-Abdely, A.M. Alkhamis, K.H. Alanazi, W.M. Banjar, O. Abdalla, M. Mohammed, H.S. Algarni, A. Alzahrani, A.A. Alsharef, R.F. Alhakeem, H.A.A. Jokhdar, A.M. Assiri); Centers for Disease Control and Prevention, Atlanta, Georgia, USA (C.M. Midgley, G.R. Abedi, X. Lu, A.M. Binder, A. Tamin, S. Lester, R.M. Dahl, S.K. Sakthivel, N.J. Thornburg, D.D. Erdman, J.T. Watson, S.I. Gerber); Princess Nourah Bint Abdulrahman University, Riyadh (W.M. Banjar); Prince Mohammed Bin Abdulaziz Hospital, Riyadh (A. Algwizani, F. Bafaqeeh, S.S. Ghazal)
Main Article
Figure 4
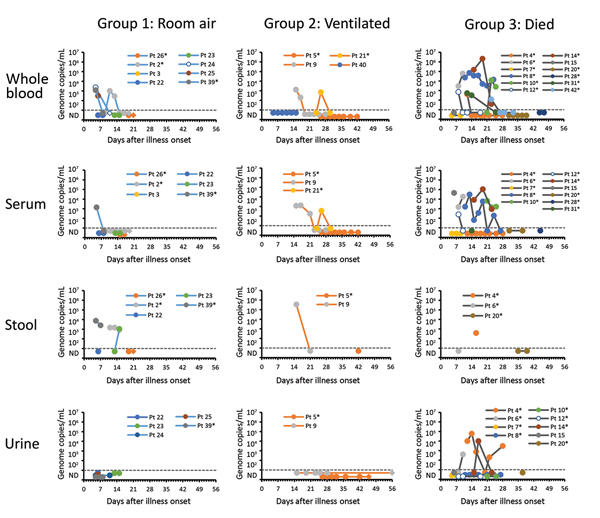
Figure 4. Estimated viral loads in non–respiratory tract specimens collected from hospitalized MERS-CoV patients, Saudi Arabia, August 1, 2015–August 31, 2016, and submitted to the US Centers for Disease Control and Prevention. Specimen types are shown by severity group. Estimated viral loads are based on upstream of the envelope (upE) real-time reverse transcription PCR cycle threshold values, or N2 cycle threshold values if the upE real-time reverse transcription PCR was negative. The dashed line represents the limit of detection, below which specimens were considered MERS-CoV–negative or not detected. Round data points represent specimens collected during the MERS-CoV detection period (defined by clinical results from respiratory specimens). Diamond data points represent specimens collected after the MERS-CoV detection period (defined by clinical results from respiratory specimens); no specimens were positive for MERS-CoV after the detection period. *Patients with a documented history of diabetes mellitus. MERS-CoV, Middle East respiratory syndrome coronavirus; Pt, patient.
Main Article
Page created: March 18, 2019
Page updated: March 18, 2019
Page reviewed: March 18, 2019
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
