Volume 25, Number 5—May 2019
Research
Serologic Prevalence of Ebola Virus in Equatorial Africa
Figure 5
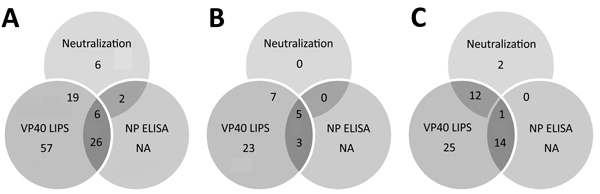
Figure 5. Overlap of different assay results for Ebola virus serology across all samples in study of serologic prevalence of Ebola virus in equatorial Africa. A) Total sample set; B) sample sets from Kinshasha, Democratic Republic of the Congo; C) sample set from Kasaï Oriental Province, Democratic Republic of the Congo. LIPS, luciferase immunoprecipitation system; NA, not applicable (ELISA was performed only for samples with positive results in other assays); NP, nucleoprotein; VP40, matrix protein.
1Current affiliation: University of Veterinary Medicine, Hannover, Germany.
Page created: April 17, 2019
Page updated: April 17, 2019
Page reviewed: April 17, 2019
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.