Volume 25, Number 9—September 2019
Research
Effect of Pneumococcal Conjugate Vaccines on Pneumococcal Meningitis, England and Wales, July 1, 2000–June 30, 2016
Figure 1
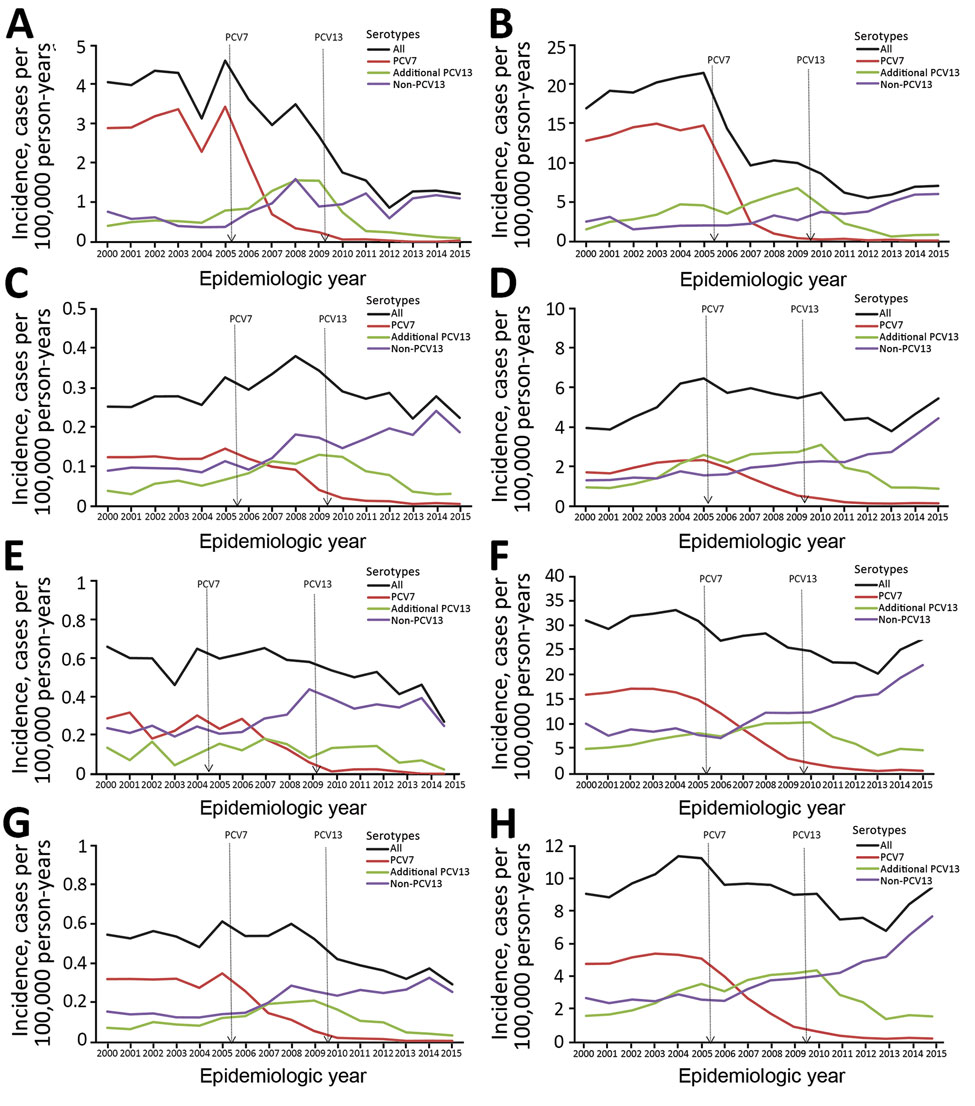
Figure 1. Corrected trends in incidence of pneumococcal meningitis and nonmeningitis cases by Streptococcus pneumoniae serotype, age group, and epidemiologic year, England and Wales, July 1, 2000–June 30, 2016. A–H) Meningitis (A, C, E, G) and nonmeningitis (B, D, F, H) cases in patients <5 years of age (A, B); patients 5–64 years of age (C, D); patients >65 years of age (E, F); and patients of all ages (G, H). The raw numbers of cases for each year were corrected for missing serotype and age with the assumption that cases with missing data for age, serotype, or both had the same age and serotype distribution as those cases for which this information was known; cases were also corrected for annual changes in population denominators in each age group (13).The vertical lines denote the introduction of PCV7 and PCV13 into the national childhood immunization program. PCV7 refers to serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F, and additional PCV13 refers to serotypes 1, 3, 5, 6A, 7F, and 19A. Non-PCV13 refers to all other serotypes. PCV7, 7-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine.
References
- Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Lynfield R, Hadler JL, et al.; Emerging Infections Programs Network. Bacterial meningitis in the United States, 1998-2007. N Engl J Med. 2011;364:2016–25. DOIPubMedGoogle Scholar
- Johnson AP, Waight P, Andrews N, Pebody R, George RC, Miller E. Morbidity and mortality of pneumococcal meningitis and serotypes of causative strains prior to introduction of the 7-valent conjugant pneumococcal vaccine in England. J Infect. 2007;55:394–9. DOIPubMedGoogle Scholar
- Stanek RJ, Mufson MA. A 20-year epidemiological study of pneumococcal meningitis. Clin Infect Dis. 1999;28:1265–72. DOIPubMedGoogle Scholar
- Oligbu G, Collins S, Sheppard CL, Fry NK, Slack M, Borrow R, et al. Childhood deaths attributable to invasive pneumococcal disease in England and Wales, 2006–2014. Clin Infect Dis. 2017;65:308–14. DOIPubMedGoogle Scholar
- Neuman HB, Wald ER. Bacterial meningitis in childhood at the Children’s Hospital of Pittsburgh: 1988-1998. Clin Pediatr (Phila). 2001;40:595–600. DOIPubMedGoogle Scholar
- Quagliarello V, Scheld WM. Bacterial meningitis: pathogenesis, pathophysiology, and progress. N Engl J Med. 1992;327:864–72. DOIPubMedGoogle Scholar
- Jit M. The risk of sequelae due to pneumococcal meningitis in high-income countries: a systematic review and meta-analysis. J Infect. 2010;61:114–24. DOIPubMedGoogle Scholar
- Braun JS, Sublett JE, Freyer D, Mitchell TJ, Cleveland JL, Tuomanen EI, et al. Pneumococcal pneumolysin and H(2)O(2) mediate brain cell apoptosis during meningitis. J Clin Invest. 2002;109:19–27. DOIPubMedGoogle Scholar
- Hoffmann O, Priller J, Prozorovski T, Schulze-Topphoff U, Baeva N, Lunemann JD, et al. TRAIL limits excessive host immune responses in bacterial meningitis. J Clin Invest. 2007;117:2004–13. DOIPubMedGoogle Scholar
- Trotter CL, Waight P, Andrews NJ, Slack M, Efstratiou A, George R, et al. Epidemiology of invasive pneumococcal disease in the pre-conjugate vaccine era: England and Wales, 1996-2006. J Infect. 2010;60:200–8. DOIPubMedGoogle Scholar
- Salisbury D, Ramsay M, Noakes K, editors. Immunisation against infectious disease. The green book. Norwich, England: The Stationery Office; 2006 [cited 2018 May 3]. https://webarchive.nationalarchives.gov.uk/20130104181824/https://www.wp.dh.gov.uk/immunisation/files/2012/09/Green-Book-updated-040113.pdf
- Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MPE, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15:535–43. DOIPubMedGoogle Scholar
- Miller E, Andrews NJ, Waight PA, Slack MPE, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011;11:760–8. DOIPubMedGoogle Scholar
- Pichon B, Ladhani SN, Slack MPE, Segonds-Pichon A, Andrews NJ, Waight PA, et al. Changes in molecular epidemiology of streptococcus pneumoniae causing meningitis following introduction of pneumococcal conjugate vaccination in England and Wales. J Clin Microbiol. 2013;51:820–7. DOIPubMedGoogle Scholar
- Ben-Shimol S, Givon-Lavi N, Grisaru-Soen G, Megged O, Greenberg D, Dagan R; Israel Bacteremia and Meningitis Active Surveillance Group. Comparative incidence dynamics and serotypes of meningitis, bacteremic pneumonia and other-IPD in young children in the PCV era: Insights from Israeli surveillance studies. Vaccine. 2018;36:5477–84. DOIPubMedGoogle Scholar
- Alari A, Chaussade H, Domenech De Cellès M, Le Fouler L, Varon E, Opatowski L, et al. Impact of pneumococcal conjugate vaccines on pneumococcal meningitis cases in France between 2001 and 2014: a time series analysis. BMC Med. 2016;14:211. DOIPubMedGoogle Scholar
- Hsu HE, Shutt KA, Moore MR, Beall BW, Bennett NM, Craig AS, et al. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N Engl J Med. 2009;360:244–56. DOIPubMedGoogle Scholar
- Ruiz-Contreras J, Picazo J, Casado-Flores J, Baquero-Artigao F, Hernández-Sampelayo T, Otheo E, et al.; HERACLES STUDY GROUP. Impact of 13-valent pneumococcal conjugate vaccine on pneumococcal meningitis in children. Vaccine. 2017;35(35 Pt B):4646–51. DOIPubMedGoogle Scholar
- Tsai CJ, Griffin MR, Nuorti JP, Grijalva CG. Changing epidemiology of pneumococcal meningitis after the introduction of pneumococcal conjugate vaccine in the United States. Clin Infect Dis. 2008;46:1664–72. DOIPubMedGoogle Scholar
- Olarte L, Barson WJ, Barson RM, Lin PL, Romero JR, Tan TQ, et al. Impact of the 13-valent pneumococcal conjugate vaccine on pneumococcal meningitis in US children. Clin Infect Dis. 2015;61:767–75. DOIPubMedGoogle Scholar
- Tsaban G, Ben-Shimol S. Indirect (herd) protection, following pneumococcal conjugated vaccines introduction: A systematic review of the literature. Vaccine. 2017;35:2882–91. DOIPubMedGoogle Scholar
- Ben-Shimol S, Greenberg D, Givon-Lavi N, Schlesinger Y, Miron D, Aviner S, et al.; Israel Bacteremia and Meningitis Active Surveillance Group. Impact of PCV7/PCV13 introduction on invasive pneumococcal disease (IPD) in young children: Comparison between meningitis and non-meningitis IPD. Vaccine. 2016;34:4543–50. DOIPubMedGoogle Scholar
- Alexandre C, Dubos F, Courouble C, Pruvost I, Varon E, Martinot A; Hospital Network for Evaluating the Management of Common Childhood Diseases. Rebound in the incidence of pneumococcal meningitis in northern France: effect of serotype replacement. Acta Paediatr. 2010;99:1686–90. DOIPubMedGoogle Scholar
- Cohen R, Biscardi S, Levy C. The multifaceted impact of pneumococcal conjugate vaccine implementation in children in France between 2001 to 2014. Hum Vaccin Immunother. 2016;12:277–84. DOIPubMedGoogle Scholar
- Imöhl M, Möller J, Reinert RR, Perniciaro S, van der Linden M, Aktas O. Pneumococcal meningitis and vaccine effects in the era of conjugate vaccination: results of 20 years of nationwide surveillance in Germany. BMC Infect Dis. 2015;15:61. DOIPubMedGoogle Scholar
- Ricketson LJ, Wood ML, Vanderkooi OG, MacDonald JC, Martin IE, Demczuk WH, et al.; Calgary Streptococcus pneumoniae Epidemiology Research (CASPER) investigators. Trends in asymptomatic nasopharyngeal colonization with streptococcus pneumoniae after introduction of the 13-valent pneumococcal conjugate vaccine in Calgary, Canada. Pediatr Infect Dis J. 2014;33:724–30. DOIPubMedGoogle Scholar
- Moreira M, Castro O, Palmieri M, Efklidou S, Castagna S, Hoet B. A reflection on invasive pneumococcal disease and pneumococcal conjugate vaccination coverage in children in Southern Europe (2009-2016). Hum Vaccin Immunother. 2017;13:1–12. DOIPubMedGoogle Scholar
- de Oliveira LH, Camacho LA, Coutinho ES, Martinez-Silveira MS, Carvalho AF, Ruiz-Matus C, et al. Impact and effectiveness of 10 and 13-valent pneumococcal conjugate vaccines on hospitalization and mortality in children aged less than 5 years in Latin American countries: a systematic review. PLoS One. 2016;11:
e0166736 . DOIPubMedGoogle Scholar - Polkowska A, Toropainen M, Ollgren J, Lyytikäinen O, Nuorti JP. Bacterial meningitis in Finland, 1995-2014: a population-based observational study. BMJ Open. 2017;7:
e015080 . DOIPubMedGoogle Scholar - Shinjoh M, Yamaguchi Y, Iwata S. Pediatric bacterial meningitis in Japan, 2013-2015 - 3-5 years after the wide use of Haemophilus influenzae type b and Streptococcus pneumoniae conjugated vaccines. J Infect Chemother. 2017;23:427–38. DOIPubMedGoogle Scholar
- Jacobs DM, Yung F, Hart E, Nguyen MNH, Shaver A. Trends in pneumococcal meningitis hospitalizations following the introduction of the 13-valent pneumococcal conjugate vaccine in the United States. Vaccine. 2017;35:6160–5. DOIPubMedGoogle Scholar
- Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, et al.; Active Bacterial Core Surveillance/Emerging Infections Program Network. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. DOIPubMedGoogle Scholar
- Verhaegen J, Vandecasteele SJ, Vandeven J, Verbiest N, Lagrou K, Peetermans WE. Antibiotic susceptibility and serotype distribution of 240 Streptococcus pneumoniae causing meningitis in Belgium 1997-2000. Acta Clin Belg. 2003;58:19–26. DOIPubMedGoogle Scholar
- Vieira AC, Gomes MC, Rolo Filho M, Eudes Filho J, Bello EJM, de Figueiredo RB. Streptococcus pneumoniae: a study of strains isolated from cerebrospinal fluid. J Pediatr (Rio J). 2007;83:71–8. DOIPubMedGoogle Scholar
- Imöhl M, Reinert RR, van der Linden M. Regional differences in serotype distribution, pneumococcal vaccine coverage, and antimicrobial resistance of invasive pneumococcal disease among German federal states. Int J Med Microbiol. 2010;300:237–47. DOIPubMedGoogle Scholar
- Richter SS, Heilmann KP, Dohrn CL, Riahi F, Diekema DJ, Doern GV. Pneumococcal serotypes before and after introduction of conjugate vaccines, United States, 1999-2011(1.). Emerg Infect Dis. 2013;19:1074–83. DOIPubMedGoogle Scholar
- Hanquet G, Kissling E, Fenoll A, George R, Lepoutre A, Lernout T, et al. Pediatric pneumococcal serotypes in 4 European countries. Emerg Infect Dis. 2010;16:1428–39. DOIPubMedGoogle Scholar
- Janoir C, Lepoutre A, Gutmann L, Varon E. Insight into resistance phenotypes of emergent non 13-valent pneumococcal conjugate vaccine type pneumococci isolated from invasive disease after 13-valent pneumococcal conjugate vaccine implementation in France. Open Forum Infect Dis. 2016;3:
ofw020 . DOIPubMedGoogle Scholar - Mahjoub-Messai F, Doit C, Koeck JL, Billard T, Evrard B, Bidet P, et al. Population snapshot of Streptococcus pneumoniae serotype 19A isolates before and after introduction of seven-valent pneumococcal Vaccination for French children. J Clin Microbiol. 2009;47:837–40. DOIPubMedGoogle Scholar
- Rückinger S, von Kries R, Siedler A, van der Linden M. Association of serotype of Streptococcus pneumoniae with risk of severe and fatal outcome. Pediatr Infect Dis J. 2009;28:118–22. DOIPubMedGoogle Scholar
- Balsells E, Guillot L, Nair H, Kyaw MH. Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: A systematic review and meta-analysis. PLoS One. 2017;12:
e0177113 . DOIPubMedGoogle Scholar - Varon E, Cohen R, Béchet S, Doit C, Levy C. Invasive disease potential of pneumococci before and after the 13-valent pneumococcal conjugate vaccine implementation in children. Vaccine. 2015;33:6178–85. DOIPubMedGoogle Scholar
- Levy C, Varon E, Béchet S, Cohen R. Effect of the 13-valent pneumococcal conjugate vaccine on pneumococcal meningitis in children. Clin Infect Dis. 2016;62:131–2. DOIPubMedGoogle Scholar
- Ricketson LJ, Conradi NG, Vanderkooi OG, Kellner JD. Changes in the nature and severity of invasive pneumococcal disease in children before and after the seven-valent and thirteen-valent pneumococcal conjugate vaccine programs in Calgary, Canada. Pediatr Infect Dis J. 2018;37:22–7.DOIPubMedGoogle Scholar
- Ladhani SN, Slack MPE, Andrews NJ, Waight PA, Borrow R, Miller E. Invasive pneumococcal disease after routine pneumococcal conjugate vaccination in children, England and Wales. Emerg Infect Dis. 2013;19:61–8. DOIPubMedGoogle Scholar
- Hirose TE, Maluf EM, Rodrigues CO. Pneumococcal meningitis: epidemiological profile pre- and post-introduction of the pneumococcal 10-valent conjugate vaccine. J Pediatr (Rio J). 2015;91:130–5. DOIPubMedGoogle Scholar
- Henderson KL, Muller-Pebody B, Ladhani S, Sharland M, Johnson AP. NICE on bacterial meningitis. Vancomycin may not be necessary. BMJ. 2010;341(aug27 2):c4704. DOIPubMedGoogle Scholar
- Henderson KL, Muller-Pebody B, Blackburn RM, Johnson AP. Reduction in erythromycin resistance in invasive pneumococci from young children in England and Wales. J Antimicrob Chemother. 2010;65:369–70. DOIPubMedGoogle Scholar