Volume 26, Number 10—October 2020
Research
Multicenter Prevalence Study Comparing Molecular and Toxin Assays for Clostridioides difficile Surveillance, Switzerland
Figure 3
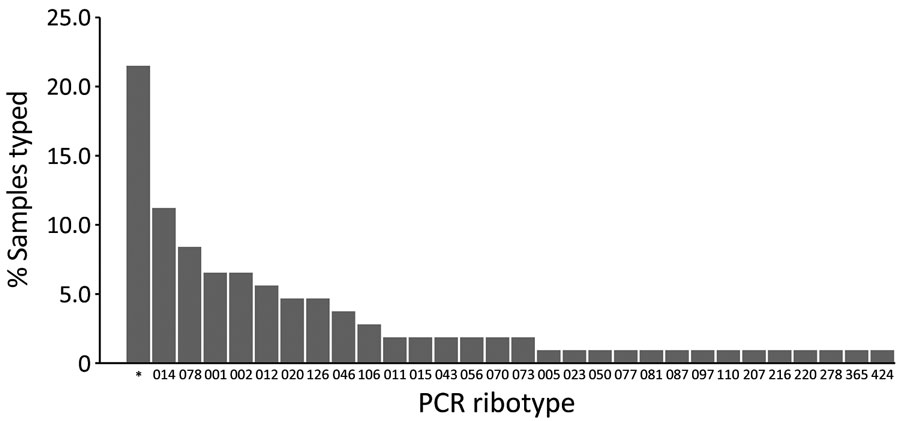
Figure 3. Distribution of PCR ribotypes among 107 samples collected in a prevalence study comparing molecular and toxin assays for nationwide surveillance of Clostridioides difficile, Switzerland. *Unknown ribotype.
Page created: July 24, 2020
Page updated: September 17, 2020
Page reviewed: September 17, 2020
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.