Volume 26, Number 3—March 2020
Research
Randomized Trial of 2 Schedules of Meningococcal B Vaccine in Adolescents and Young Adults, Canada1
Figure 2
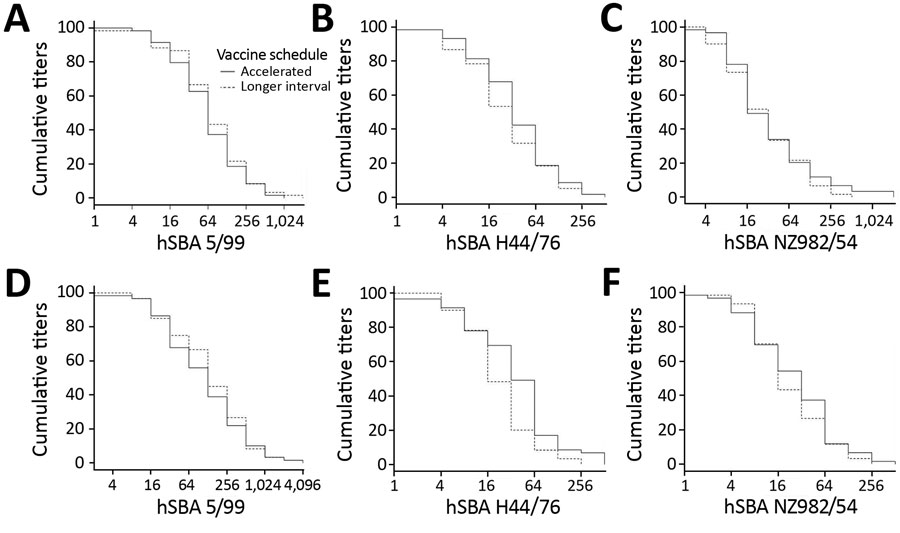
Figure 2. Reverse cumulative distribution curves of hSBA titers to 3 vaccine strains in recipients in trial of 4-component protein-based meningococcal B vaccine administered at 0 and 21 days compared with 0 and 60 days, Canada. A), B), and C) Comparisons made at day 21. D), E), and F) Comparisons made at day 180. hSBA, human serum bactericidal antibody; hSBA 5/99, Neisserial adhesin A surface proteins; hSBA H44/76, factor H binding protein; hSBA 982/54, New Zealand outer membrane vesicle.
1Preliminary results from this study were presented at IDWeek, October 26–30, 2016, New Orleans, LA, USA; and at the Meningitis Research Foundation Conference, November 14–15, 2017, London, England, UK.
Page created: February 19, 2020
Page updated: February 19, 2020
Page reviewed: February 19, 2020
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.