Volume 26, Number 9—September 2020
Research Letter
Leuconostoc lactis and Staphylococcus nepalensis Bacteremia, Japan
Cite This Article
Citation for Media
Abstract
Leuconostoc lactis is a glycopeptide-resistant, gram-positive, facultative anaerobic coccus isolated from dairy products, whereas Staphylococcus nepalensis is coagulase-negative coccus that has not been identified as human pathogen. We report an instructive case of L. lactis and S. nepalensis bacteremia in a 71-year-old man who experienced Boerhaave syndrome after a meal.
Leuconostoc lactis is an intrinsically glycopeptide-resistant but ampicillin-susceptible, gram-positive, facultative anaerobic coccus (1) found in food products including dairy products, vegetables, and wine. L. lactis is a very rare pathogen associated with bloodstream infections (2). Staphylococcus nepalensis is a novobiocin-resistant coagulase-negative staphylococcus also found in food products, such as dry-cured ham and fish sauce, that has not been reported as a human pathogen (3–5). Neither L. lactis nor S. nepalensis is part of normal human bacterial flora (2,3).
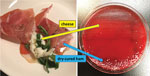
A 71-year-old man with hypertension and hyperlipidemia sought care for upper abdominal pain and vomiting after a meal at his son’s restaurant. A computed tomography (CT) scan showed collapse of the lower esophagus wall and expansion of the mediastinum; medical staff diagnosed a spontaneous esophageal rupture and performed emergency surgery. Surgical findings demonstrated a 5 cm perforation of the lower esophagus with no rupture to the thoracic and abdominal cavity. The final diagnosis included Boerhaave syndrome, esophageal hiatus hernia, and mediastinitis. Two sets of blood culture taken on day 1 were positive for gram-positive cocci, which we identified by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry as L. lactis in an aerobic bottle (10.7 h to culture) and an anaerobic bottle (13.3 h to culture) and S. nepalensis in 1 anaerobic bottle (24.3 h to culture). The 2 bacteria were indications of true bacteremia; therefore, we escalated ampicillin/sulbactam (treatment to piperacillin/tazobactam for L. lactis (Appendix Table 1) and initiated vancomycin treatment for S. nepalensis on day 3 after admission (Appendix Table 2). We measured MICs in the microdilution method using the MicroScan WalkAway 96 SI system (Beckman Coulter, https://www.beckmancoulter.com) with a MICrofast7J panel and determined the susceptibility of L. lactis according to Clinical and Laboratory Standards Institute (CLSI) guidelines (6). On day 7, we deescalated piperacillin/tazobactam to ampicillin/sulbactam, referring to the MICs, and we obtained follow-up sets of blood culture. The culture results were negative. We discontinued vancomycin by day 14 but maintained the ampicillin/sulbactam regimen. A follow-up CT scan on day 28 showed a subsiding mediastinal abscess. Moreover, a pathological examination of the surgical biopsy demonstrated no esophageal cancer. On the basis of the clinical course of the disease, we strongly suspected a breakthrough of L. lactis and S. nepalensis through the ruptured esophagus into the bloodstream. To prove this relationship, we obtained permission from the patient’s son to analyze samples of the food products his father consumed, including cheese, dry-cured ham, sauerkraut, pizza margherita, bianchetti (pasta with boiled young sardines), and red and white wine. We cultured samples from these products on blood agar medium; colonies of L. lactis, confirmed by MALDI-TOF mass spectrometry, were derived from cheese samples (Figure).
Approximately 20 cases of L. lactis bacteremia have been reported (1), mostly in immunosuppressed patients with malignancy including leukemia, diabetes, or impaired skin barrier function due to central venous catheter. Several entry routes to the bloodstream have been hypothesized, including the digestive tract or the skin in catheter-related bloodstream infections, or as a result of microbial substitution due to glycopeptide administration; however, no entry point has been definitively identified (1,2,7,8). In addition, L. lactis bacteremia caused by gastrointestinal tract perforation had not been reported. We concluded that L. lactis colonized cheese and entered the bloodstream through a perforation of the lower esophagus, and we were able to demonstrate that L. lactis can enter the bloodstream through a rupture of the digestive tract. Based on our findings, we may advise screening for gastrointestinal diseases, such as ulcer, perforation, and malignancy, in patients with L. lactis bacteremia.
S. nepalensis has not previously been reported as a human pathogen, nor has its pathogenicity been described. Because the results of the food sample cultures identified other coagulase-negative Staphylococci bacteria (S. equorum and S. xylosus), rather than S. nepalensis, from the dry-cured ham colonies, we could not conclusively demonstrate the entry of S. nepalensis to the bloodstream. Moreover, contamination with S. nepalensis was possible; only 1 anaerobic bottle of blood culture taken at admission was positive. However, because S. nepalensis is not normally found in the human microbial flora but is a part of the predominant flora in dry-cured ham together with S. equorum and S. xylosus (4), we suspect that the S. nepalensis bacteremia diagnosis was correct.
In conclusion, we demonstrate the point of entry for L. lactis into the human bloodstream and show results implying that L. lactis can be a pathogen of bacteremia, as previous reports have shown (1,2,7,8). Our report is also a suspected case of S. nepalensis bacteremia; further investigation is needed for confirmation.
Dr. Hosoya was a junior resident of the Department of Disease Control and Prevention Center, National Center for Global Health and Medicine, Tokyo, Japan at the time of this work. He is an obstetrician-gynecologist with the National Center for Global Health and Medicine. His primary research interest is infectious disease in obstetrics and gynecology.
References
- Matsuda K, Koya J, Toyama K, Ikeda M, Arai S, Nakamura F, et al. A therapeutic benefit of daptomycin against glycopeptide-resistant gram-positive cocci bloodstream infections under neutropenia. J Infect Chemother. 2017;23:788–90. DOIPubMedGoogle Scholar
- Yang C, Wang D, Zhou Q, Xu J. Bacteremia due to vancomycin-resistant Leuconostoc lactis in a patient with pneumonia and abdominal infection. Am J Med Sci. 2015;349:282–3. DOIPubMedGoogle Scholar
- Nováková D, Pantůcek R, Petrás P, Koukalová D, Sedlácek I. Occurance of Staphylococcus nepalensis strains in different sources including human clinical material. FEMS Microbiol Lett. 2006;263:163–8. DOIPubMedGoogle Scholar
- Fulladosa E, Garriga M, Martín B, Guàrdia MD, García-Regueiro JA, Arnau J. Volatile profile and microbiological characterization of hollow defect in dry-cured ham. Meat Sci. 2010;86:801–7. DOIPubMedGoogle Scholar
- Fukami K, Satomi M, Funatsu Y, Kawasaki K, Watabe S. Characterization and distribution of Staphylococcus sp. implicated for improvement of fish sauce odor. Fish Sci. 2004;70:916–23. DOIGoogle Scholar
- Clinical and Laboratory Standards Institute. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria. 3rd ed. CLSI guideline M45. Wayne (PA): The Institute; 2016.DOIPubMedGoogle Scholar
- Lee MR, Huang YT, Lee PI, Liao CH, Lai CC, Lee LN, et al. Healthcare-associated bacteraemia caused by Leuconostoc species at a university hospital in Taiwan between 1995 and 2008. J Hosp Infect. 2011;78:45–9. DOIPubMedGoogle Scholar
- Patel T, Molloy A, Smith R, Balakrishnan I. Successful treatment of Leuconostoc bacteremia in a neutropenic patient with tigecycline. Infect Dis Rep. 2012;4:
e31 . DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: July 27, 2020
Table of Contents – Volume 26, Number 9—September 2020
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Satoshi Kutsuna, Department of Disease Control and Prevention Center, National Center for Global Health and Medicine, 1-21-1, Toyama, Shinjuku-ku, Tokyo 162-8655, Japan
Top