Volume 27, Number 1—January 2021
Research
Post–13-Valent Pneumococcal Conjugate Vaccine Dynamics in Young Children of Serotypes Included in Candidate Extended-Spectrum Conjugate Vaccines
Figure 1
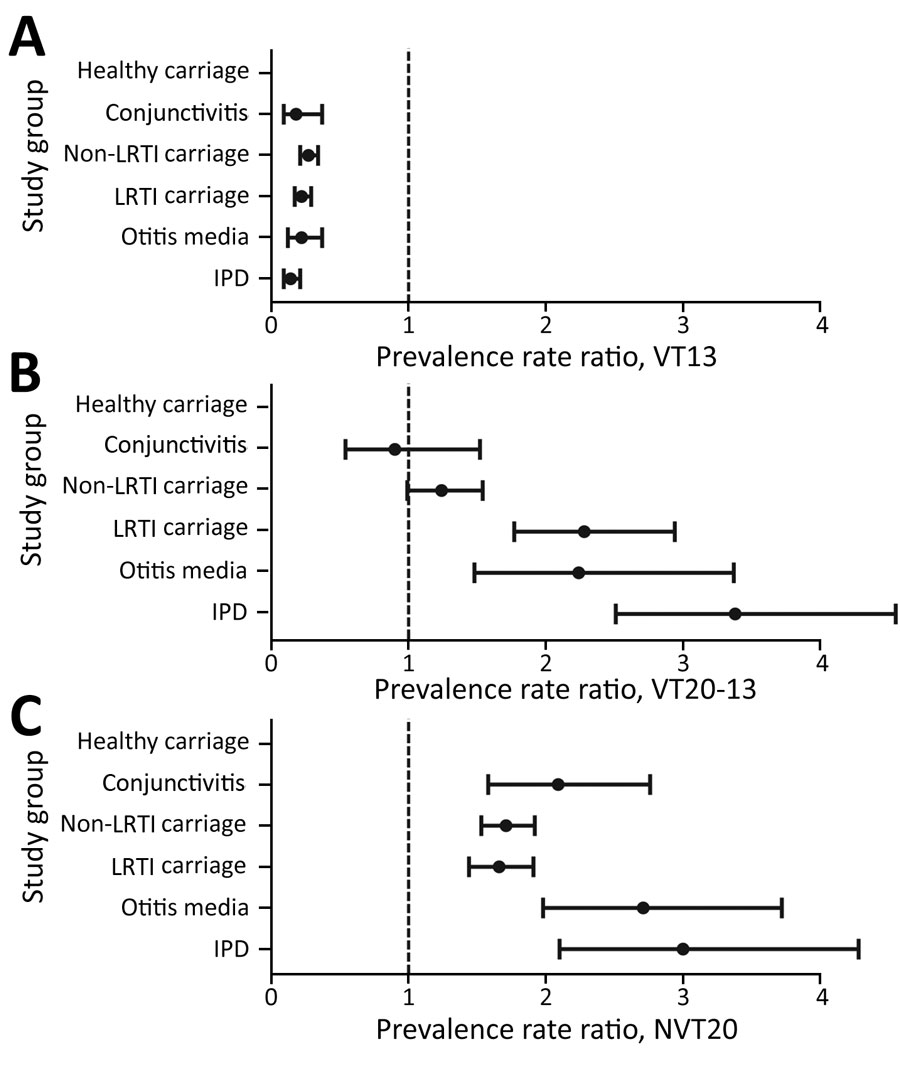
Figure 1. Postvaccine dynamics of pneumococcal conjugate vaccines in children <24 months of age, Israel, July 2009–June 2017. A) Healthy children; B) children with non–LRTIs; C) children with lower respiratory infections; D) children with conjunctivitis; E) children with otitis media (isolates from middle ear fluid were tested); and F) children with invasive pneumococcal disease (isolates from blood and cerebrospinal fluid were tested). Data show VT13, VT20–13, and NVT20 as the proportion of each serotype of all pneumococcal isolates. p<0.05 for all comparisons comparing 2015–2017 versus 2009–2011, except for VT20–13 in conjunctivitis. LRTI, lower respiratory tract infection; NVT, nonvaccine serotype; VT, vaccine serotype.
Page created: November 19, 2020
Page updated: December 21, 2020
Page reviewed: December 21, 2020
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.