Volume 27, Number 1—January 2021
Dispatch
Limited Specificity of Serologic Tests for SARS-CoV-2 Antibody Detection, Benin
Figure 1
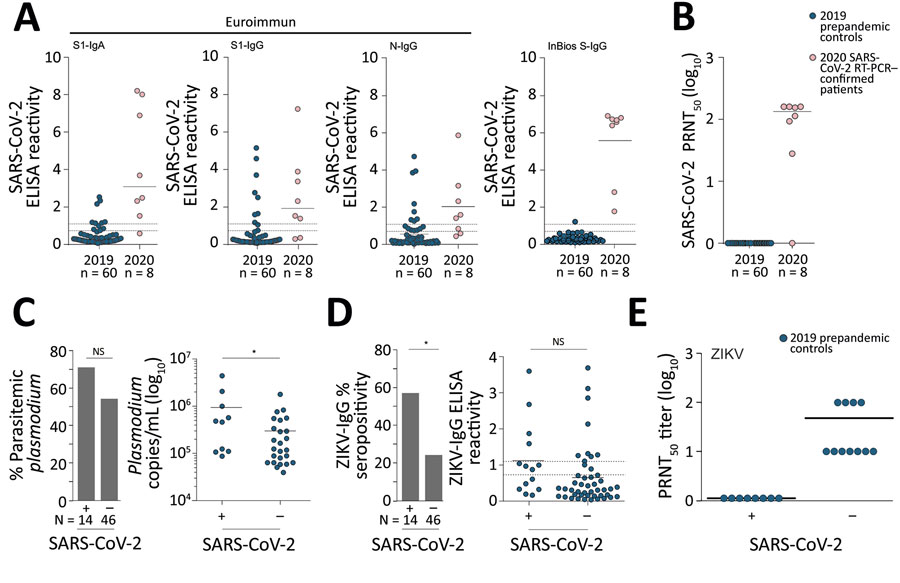
Figure 1. Serologic diagnostics of SARS-CoV-2 and co-existing pathogens in Benin. A) SARS-CoV-2 ELISA reactivity by using different commercially available assays in prepandemic controls from 2019 and SARS-CoV-2 RT-PCR-confirmed patients from 2020. Dashed lines denote the ratio thresholds of >1.1 (positive) and <0.9 (negative); results between these values are considered borderline, as defined by the manufacturers, EUROIMMUN (https://www.euroimmun.com) and InBios (https://inbios.com). Solid line denotes mean ELISA reactivity. B) SARS-CoV-2 PRNT50 in prepandemic controls from 2019 and SARS-CoV-2 RT-PCR–confirmed patients from 2020, shown in log10 scale for clarity. Solid line denotes mean PRNT log10 titer. C) Percentage of prepandemic controls with Plasmodium parasitemia who were SARS-CoV-2 ELISA–positive versus those who were SARS-CoV-2 ELISA-negative, shown in log10 scale for clarity. Solid line denotes the mean copies/mL. Asterisk denotes p<0.05. D) ZIKV ELISA IgG ELISA percent seropositivity and ZIKV ELISA reactivity within SARS-CoV-2–positive and SARS-CoV-2–negative prepandemic controls. Continuous line denotes the mean ELISA reactivity. Asterisk denotes p<0.05. E) ZIKV PRNT50 results. Continuous line denotes the mean PRNT50 log10 reactivity. NS, not statistically significant; PRNT50, 50% plaque reduction neutralization test; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ZIKV, Zika virus.
1These first authors contributed equally to this article.
2These senior authors contributed equally to this article.