Volume 27, Number 12—December 2021
Research
Novel Assay to Measure Seroprevalence of Zika Virus in the Philippines
Figure 3
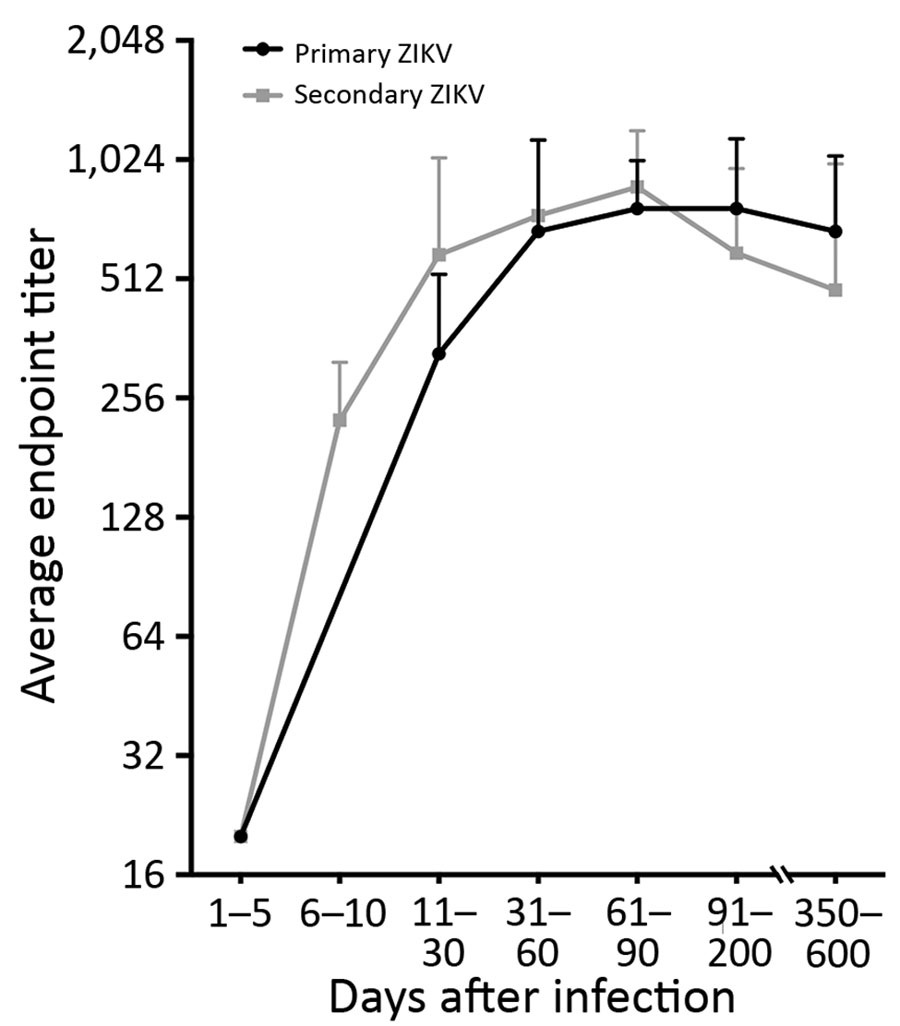
Figure 3. Durability of ZIKV EDIII antibodies in study of novel assay to measure ZIKV seroprevalence in the Philippines. Longitudinal samples from patients with PCR-confirmed ZIKV infections were collected 1–600 days after symptom onset and tested for ZIKV EDIII–binding antibodies. DENV serostatus was determined by DENV focus reduction neutralization test. Primary ZIKV serostatus indicates ZIKV infection in DENV-naive participants; secondary ZIKV serostatus indicates ZIKV infection in participants previously infected with DENV. DENV, dengue virus; EDIII, E protein domain III; ZIKV, Zika virus.
1Deceased.
Page created: August 25, 2021
Page updated: November 19, 2021
Page reviewed: November 19, 2021
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.