Volume 27, Number 2—February 2021
Research Letter
Long-Term Humoral Immune Response in Persons with Asymptomatic or Mild SARS-CoV-2 Infection, Vietnam
Figure
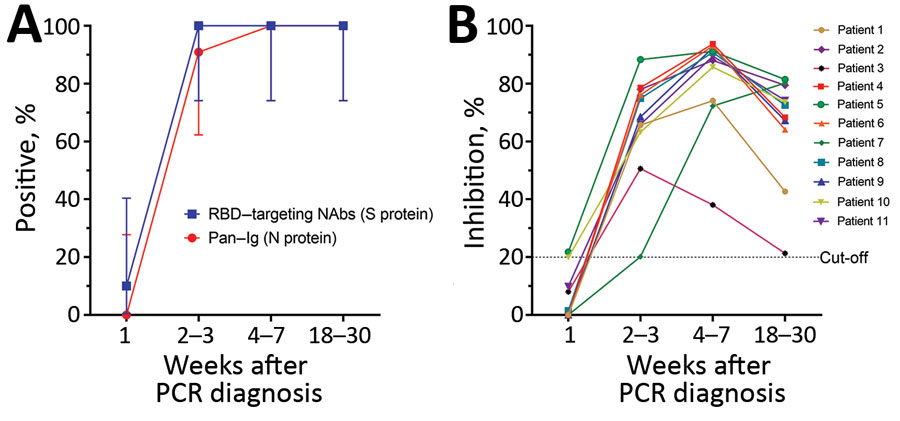
Figure. Antibody responses in 11 study participants, weeks 1–20 after PCR diagnosis of SARS-CoV-2 infection, Vietnam, 2020. A) Seroprevalence of SARS-CoV-2 among 11 COVID-19 patients. We followed testing protocols and the positive cutoff of 20% recommended in the Elecsys Anti–SARS-CoV-2 assay (Roche, https://diagnostics.roche.com) without any modification. Using these parameters, previous studies showed an excellent concordance between results from surrogate virus neutralization tests and conventional neutralizing antibody detection assays (3,4). Vertical bars denote 95% CIs. Graphs were created using GraphPad Prism version 8.0 (GraphPad software, https://www.graphpad.com). B) Kinetics of neutralizing antibodies measured by the surrogate neutralization assay (GenScript, https://www.genscript.com) with the 20% cutoff applied. We tested samples at 1:10 dilution as specified. Because of the limited availability of plasma samples, each sample was tested only once. RBD, receptor-binding domain; NAbs, neutralizing monoclonal antibodies; S, spike; N, nucleocapsid.
References
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al.; China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–33. DOIPubMedGoogle Scholar
- Chen SY, Lee YL, Lin YC, Lee NY, Liao CH, Hung YP, et al. Multicenter evaluation of two chemiluminescence and three lateral flow immunoassays for the diagnosis of COVID-19 and assessment of antibody dynamic responses to SARS-CoV-2 in Taiwan. Emerg Microbes Infect. 2020;9:2157–68. DOIPubMedGoogle Scholar
- Tan CW, Chia WN, Qin X, Liu P, Chen MI, Tiu C, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020;38:1073–8. DOIPubMedGoogle Scholar
- Perera RAPM, Ko R, Tsang OTY, Hui DSC, Kwan MYM, Brackman CJ, et al. Evaluation of a SARS-CoV-2 surrogate virus neutralization test for detection of antibody in human, canine, cat and hamster sera. J Clin Microbiol. 2020;
JCM.02504-20 . DOIPubMedGoogle Scholar - Gudbjartsson DF, Norddahl GL, Melsted P, Gunnarsdottir K, Holm H, Eythorsson E, et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383:1724–34. DOIPubMedGoogle Scholar
- Sun B, Feng Y, Mo X, Zheng P, Wang Q, Li P, et al. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg Microbes Infect. 2020;9:940–8. DOIPubMedGoogle Scholar
- Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, et al.; Oxford COVID Vaccine Trial Group. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–78. DOIPubMedGoogle Scholar
- van Doremalen N, Lambe T, Spencer A, Belij-Rammerstorfer S, Purushotham JN, Port JR, et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586:578–82. DOIPubMedGoogle Scholar
- Deng W, Bao L, Liu J, Xiao C, Liu J, Xue J, et al. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science. 2020;369:818–23. DOIPubMedGoogle Scholar