Volume 27, Number 4—April 2021
Synopsis
Difficulties in Differentiating Coronaviruses from Subcellular Structures in Human Tissues by Electron Microscopy
Figure 2
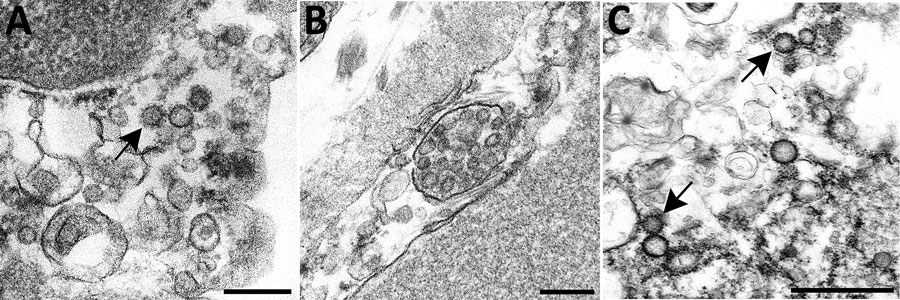
Figure 2. Overview of differential ultrastructural features of subcellular structures commonly misidentified as coronaviruses; all were prepared by thin section from formalin-fixed autopsy specimens. A) Clathrin-coated vesicles (CCVs), circular vesicles with a fringe of clathrin protein (arrow), in the cell cytoplasm range in size from 60 nm−100 nm. Differentiation: clathrin surrounding the vesicle may be misinterpreted as viral spikes, however, CCVs are free in the cell cytoplasm, and clathrin is in direct contact with the cytoplasm. Intracellular coronaviruses are found within membrane-bound vacuoles, and spikes, if visible, are in contact with the vacuolar contents. CCVs lack the internal black dots that signify cross sections through the viral nucleocapsid. Scale bar indicates 200 nm. B) Multivesicular body (MVB), a collection of membrane-bound roughly spherical vesicles formed by the inward budding of an endosomal membrane. Differentiation: MVBs may be confused with a vacuolar accumulation of coronavirus particles. Vesicles within multivesicular bodies do not have internal black dots that signify cross sections through the viral nucleocapsid. Scale bar indicates 200 nm. C) Circular cross sections through rough endoplasmic reticulum (RER) (arrows) found free within the cytoplasm. Differentiation: ribosomes along the endoplasmic reticulum may be confused with viral spikes. Ribosomes of vesiculating RER are in direct contact with the cell cytoplasm, unlike coronavirus spikes, which would be in contact with vacuolar contents. Vesiculating RER lacks cross sections through the viral nucleocapsid. Scale bar indicates 1 µm.