Volume 28, Number 10—October 2022
Dispatch
Anisakiasis Annual Incidence and Causative Species, Japan, 2018–2019
Cite This Article
Citation for Media
Abstract
Using data from 2018–2019 health insurance claims, we estimated the average annual incidence of anisakiasis in Japan to be 19,737 cases. Molecular identification of larvae revealed that most (88.4%) patients were infected with the species Anisakis simplex sensu stricto. Further insights into the pathogenesis of various anisakiasis forms are needed.
Anisakiasis is a foodborne zoonosis caused by ingestion of raw or undercooked fish and cephalopods parasitized by anisakid nematode larvae. As seafood consumption has increased globally (1), the incidence of anisakiasis has also increased worldwide (2,3). The population of Japan traditionally consumes large quantities of seafood (4), and consuming raw seafood, such as sushi and sashimi, is common.
In response to the large number of annual cases, the government of Japan added anisakiasis under food poisoning in its Ordinance for Enforcement of the Food Sanitation Act to strengthen countermeasures in 1999, when food poisoning statistics included a case of anisakiasis (5). In 2012, the government amended the ordinance and registered anisakid nematodes, comprising Anisakis spp. and Pseudoterranova spp., as a single disease agent, Anisakis, in the list of food poisoning agents (6). These measures enabled the government to aggregate the number of Anisakis food poisoning cases, which showed a near-constant increase. However, epidemiologic studies have indicated that the number of food poisoning cases in Japan is considerably higher than those officially reported (7). For anisakiasis, the discrepancy between food poisoning statistics and the actual incidence is unclear because no nationwide investigation into anisakiasis incidence has been conducted since the 2012 amendment.
We analyzed a large database of health insurance claim data (8) to estimate the national incidence of anisakiasis and determine the degree of discrepancy between foodborne statistics and actual incidence. Furthermore, we performed molecular identification of anisakid larvae isolated from infected patients to clarify the causative agent and adduce reasons for the high incidence of anisakiasis in Japan.
To estimate the number of anisakiasis patients, we used an anonymized health insurance claim database from JMDC, Inc. (https://www.jmdc.co.jp), a commercial medical database provider in Japan. The nation has a universal health insurance system wherein medical institutions prepare health insurance claims that list the name of the disease or injury on a service basis. Medical expenses, other than a portion directly paid by patients, are reimbursed from taxes and mandatory insurance fees. In this process, medical institutions first submit health insurance claims to a specialized organization that assesses the appropriateness of treatment and amount of reimbursement. In this study, we used a database covering ≈8.43 million persons annually, accounting for >6% of the total population of Japan, during January 2018–December 2019. We extracted health insurance claims with the diagnosis code B81.0 in the International Classification of Diseases, 10th Revision (ICD-10), indicating Anisakis and Pseudoterranova infections.
The number of patients with anisakiasis registered in the target database was 991 in 2018 and 766 in 2019. However, the data revealed a male-biased sex ratio and a workforce-biased distribution for persons 20–39 years of age. Therefore, we adjusted the value in each group for estimation by stratifying the population of Japan by sex and age by using a previously described expanded estimate method (9), and data from the national census conducted by the Ministry of Internal Affairs and Communications in 2015 (https://www.stat.go.jp/english/data/kokusei/2015/final_en/final_en.html). On the basis of our adjustment, we estimate the number of patients in Japan with anisakiasis was 21,511 in 2018 and 17,962 in 2019. The number of patients with anisakiasis recorded in the food poisoning statistics during the same period was considerably lower, <1/40, in our estimation (Table 1).
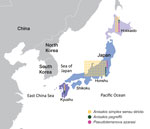
For molecular identification, we obtained 189 larvae of anisakid nematodes from BML, Inc. (http://www.bml.co.jp/eng), a privately-owned clinical testing laboratory in Japan. The larvae were isolated from 181 anisakiasis patients in 30 of 47 prefectures in Japan during 2018–2019 (Figure 1). We extracted DNA samples from individual larvae, used PCR to amplify the internal transcribed spacer 1 region, and sequenced the amplicons to distinguish between nematode genera and identify the Anisakis species. For Pseudoterranova larvae, we further amplified a portion of the NADH dehydrogenase subunit 1 gene in mitochondrial DNA and sequenced to discriminate between species of this genus (Appendix).
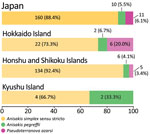
Consequently, we identified 168 (88.9%) Anisakis simplex sensu stricto larvae, 10 (5.3%) A. pegreffii larvae, and 11 (5.8%) Pseudoterranova azarasi larvae (Table 2). These findings represent perfect sequence identity with the respective species, regardless of the targets. We deposited the obtained sequences in GenBank under accession nos. LC684518–21 (Appendix Table 1).
A recent study estimated 7,700–8,320 annual anisakiasis cases in Spain, a country with a high incidence of anisakiasis in Europe (10). Although our survey methods differed, we demonstrated that Japan had an average of 19,737 anisakiasis cases per year during 2018–2019. As preventive measures, the government of Japan has repeatedly instructed local establishments (e.g., restaurants, fish mongers, and grocery stores) and consumers to freeze seafood at −20°C for at least 24 hours before consuming it raw or to remove anisakid nematodes during cooking. The Ministry of Health, Labour and Welfare of Japan provides web-based food poisoning statistics with information regarding fish species reported by anisakiasis patients and preparation procedures associated with infections in Japanese to help consumers and fishmongers avoid anisakiasis.
In Japan, A. simplex s.s. nematodes are responsible for the highest incidence of anisakiasis, whereas the species A. pegreffii is the leading cause of anisakiasis in Europe and South Korea (2). A. simplex s.s. nematodes penetrate the muscles of various fish species at a higher rate than A. pegreffii (11), which could partly explain the smaller proportion of A. pegreffii anisakiasis cases in Japan because A. pegreffii nematodes are often removed with fish viscera during the preparation of sushi and sashimi. Furthermore, fish habitat can corroborate the difference in predominant anisakid nematode species between South Korea and Japan; A. simplex s.s.–carrying fish are predominant in the Pacific side of Japan, whereas A. pegreffii–carrying fish are more common in the Sea of Japan and the East China Sea, located between South Korea and Japan (11) (Figure 1).
In this study, we identified 11 P. azarasi larvae (5.8%) from 11 patients, 6 of whom lived in Hokkaido, the northernmost insular prefecture of Japan, where cold water fish, such as Pacific cod (Gadus macrocephalus), are commonly consumed (Figures 1,2) (12). Although patients have been reported to orally expel Pseudoterranova larvae that have developed to the fourth stage (13), most (7/11 cases) P. azarasi larvae detected in this study were in the stomach.
Anisakid larvae were isolated from the stomach or intestines of 177 patients, 23 (13%) of whom were asymptomatic. These asymptomatic cases were found incidentally during health check-ups or routine follow-up cancer screening. Some studies have already reported asymptomatic gastrointestinal cases (14,15), but our findings revealed a varied and greater number of anisakid species associated with asymptomatic infections: A. simplex s.s. (91.3%), A. pegreffii (4.3%), and P. azarasi (4.3%). One urticaria case was associated with symptomatic P. azarasi gastric infection. Numerous cases of Anisakis allergy, including cases of urticaria, angioedema, and anaphylaxis, have been reported in Europe (10). In healthcare claims from Japan, Anisakis allergy was not registered as a disease name until 2021. Thus, the database did not include anisakiasis patients with allergic symptoms alone, which is a limitation of this study.
In conclusion, Anisakis infection is currently drawing more attention in Japan. Elucidating the immunopathological mechanisms behind asymptomatic and symptomatic anisakiasis is imperative and can provide insights into the pathogenesis of gastrointestinal anisakiasis.
Dr. Sugiyama is the former chief of the Laboratory of Helminthology, Department of Parasitology at the National Institute of Infectious Diseases in Tokyo, Japan, and currently continues his research in this institution as a guest researcher. His primary research interests are epidemiology and laboratory diagnosis of foodborne helminthic zoonoses, including anisakiasis and paragonimiasis.
Acknowledgments
We thank Chisato Kagawa for her support. We also thank the members of Inspection and Safety Division, Department of Food Safety, Ministry of Health, Labour and Welfare, Japan, for their assistance in re-confirming the cases of anisakiasis in the food poisoning statistics of Japan.
This study was supported by a grant from the Japan Agency for Medical Research and Development (grant no. 21fk0108136 to H.S.).
References
- Naylor RL, Kishore A, Sumaila UR, Issifu I, Hunter BP, Belton B, et al. Blue food demand across geographic and temporal scales. Nat Commun. 2021;12:5413. DOIPubMedGoogle Scholar
- Lim H, Jung BK, Cho J, Yooyen T, Shin EH, Chai JY. Molecular diagnosis of cause of anisakiasis in humans, South Korea. Emerg Infect Dis. 2015;21:342–4. DOIPubMedGoogle Scholar
- Shamsi S, Butcher AR. First report of human anisakidosis in Australia. Med J Aust. 2011;194:199–200. DOIPubMedGoogle Scholar
- Food and Agriculture Organization of the United Nations. FAO yearbook. Fishery and aquaculture statistics 2019. Rome: The Organization; 2021. DOIGoogle Scholar
- Sugiyama H. [Food and parasitic infections] [in Japanese]. Shokuhin Eiseigaku Zasshi. 2010;51:285–91. DOIPubMedGoogle Scholar
- Director-General, Department of Food Safety, Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare. Partial revision of the ordinance for enforcement of the Food Sanitation Act [in Japanese]. 2012 Dec 28; Notification shokuan no. 1228–7 [cited 2022 Apr 11]. https://www.mhlw.go.jp/topics/bukyoku/iyaku/syoku-anzen/gyousei/dl/121228_2.pdf.
- Kubota K, Kasuga F, Iwasaki E, Inagaki S, Sakurai Y, Komatsu M, et al. Estimating the burden of acute gastroenteritis and foodborne illness caused by Campylobacter, Salmonella, and Vibrio parahaemolyticus by using population-based telephone survey data, Miyagi Prefecture, Japan, 2005 to 2006. J Food Prot. 2011;74:1592–8. DOIPubMedGoogle Scholar
- Kimura S, Sato T, Ikeda S, Noda M, Nakayama T. Development of a database of health insurance claims: standardization of disease classifications and anonymous record linkage. J Epidemiol. 2010;20:413–9. DOIPubMedGoogle Scholar
- Ohisa M, Kimura Y, Matsuo J, Akita T, Sato T, Matsuoka T, et al. Estimated numbers of patients with liver disease related to hepatitis B or C virus infection based on the database reconstructed from medical claims from 2008 to 2010 in Japan. Hepatol Res. 2015;45:1228–40. DOIPubMedGoogle Scholar
- Bao M, Pierce GJ, Pascual S, González-Muñoz M, Mattiucci S, Mladineo I, et al. Assessing the risk of an emerging zoonosis of worldwide concern: anisakiasis. Sci Rep. 2017;7:43699. DOIPubMedGoogle Scholar
- Suzuki J, Murata R, Kodo Y. Current status of anisakiasis and Anisakis larvae in Tokyo, Japan. Food Saf (Tokyo). 2021;9:89–100. DOIPubMedGoogle Scholar
- Arizono N, Miura T, Yamada M, Tegoshi T, Onishi K. Human infection with Pseudoterranova azarasi roundworm. Emerg Infect Dis. 2011;17:555–6. DOIPubMedGoogle Scholar
- Torres P, Jercic MI, Weitz JC, Dobrew EK, Mercado RA. Human pseudoterranovosis, an emerging infection in Chile. J Parasitol. 2007;93:440–3. DOIPubMedGoogle Scholar
- Nakaji K, Kumamoto M, Wada Y, Nakae Y. Asymptomatic gastric and colonic anisakiasis detected simultaneously. Intern Med. 2019;58:2263–4. DOIPubMedGoogle Scholar
- Song H, Jung BK, Cho J, Chang T, Huh S, Chai JY. Molecular identification of Anisakis larvae extracted by gastrointestinal endoscopy from health check-up patients in Korea. Korean J Parasitol. 2019;57:207–11. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: September 15, 2022
Table of Contents – Volume 28, Number 10—October 2022
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Hiromu Sugiyama, Department of Parasitology, National Institute of Infectious Diseases, Toyama 1-23-1, Shinjuku, Tokyo, 162-8640, Japan
Top