Volume 28, Number 11—November 2022
Dispatch
Seroincidence of Enteric Fever, Juba, South Sudan
Cite This Article
Citation for Media
Abstract
We applied a new serosurveillance tool to estimate typhoidal Salmonella burden using samples collected during 2020 from a population in Juba, South Sudan. By using dried blood spot testing, we found an enteric fever seroincidence rate of 30/100 person-years and cumulative incidence of 74% over a 4-year period.
Enteric fever, caused by Salmonella enterica serovars Typhi and Paratyphi, causes substantial illness and death globally (1). However, estimating the population-level burden of infection is challenging. Blood culture, the standard for both diagnosis and surveillance, requires microbiological laboratory facilities that are not available in many low- and-middle-income countries. Challenges in accessing blood culture, along with an estimated diagnostics sensitivity of only 60% (2), contribute to chronic underdetection (3).
Juba, the capital of South Sudan, experiences a high burden of enteric infections such as cholera and hepatitis E virus (4,5). Enteric fever is a frequently diagnosed etiology of acute fever, but few laboratories have blood culture capacity for confirmation. Consequentially, the population-level burden of enteric fever is unknown.
Hemolysin E (HlyE), a pore-forming toxin, is a sensitive and specific serologic marker for diagnosing typhoidal Salmonella (6–10) and is not associated with typhoid carriage (11). New serologic and analytic tools enable measurement of population-level enteric fever incidence from cross-sectional serosurveys using HlyE IgG and IgA (12). We applied those tools to generate population-level enteric fever seroincidence estimates in Juba.
We used dried blood spots (DBS) collected for a SARS-CoV-2 serosurvey in Juba, South Sudan, enrolled during August 7–September 20, 2020; enrollment and sampling methods are described elsewhere (13). In brief, 2-stage cluster sampling was used to randomly select households from predefined enumeration units from 6 administrative divisions within and surrounding Juba; all persons >1 year of age and residing for >1 week within the sampled household were eligible to participate. Capillary blood was collected onto Whatman 903 Protein Saver cards (Sigma-Aldrich, https://www.sigmaaldrich.com), air dried, and transported at ambient temperature to Massachusetts General Hospital (Boston, MA, USA), where they were stored at 4°C. We tested all banked samples collected from participants <25 years of age and a random sample of participants >25 years of age. Younger participants were prioritized because they matched the age distribution of typhoid case data used for the seroincidence estimation (12). The study protocol was approved by ethical review boards with the South Sudan Ministry of Health and Massachusetts General Hospital.
We used kinetic ELISAs to quantify HlyE IgA and IgG levels in eluted DBS as described (7,11). To estimate seroincidence, we used the antibody dynamics from a longitudinal cohort of 1,420 blood culture–confirmed enteric fever cases (12). In brief, we created a likelihood function for observed cross-sectional population antibody response data based on antibody dynamics after blood-culture confirmed infection. We generated joint incidence estimates by combining the likelihood for HlyE IgA and IgG for each age stratum using age-specific antibody dynamics. We selected age strata to match incidence estimates from blood culture enteric fever surveillance studies in other countries in sub-Saharan Africa and South Asia (14,15). This method incorporates heterogeneity in antibody responses and explicitly accounts for measurement error and biologic noise (12; Appendix reference 16).
We used 3 US populations to define the distribution of biologic noise (nonspecific antibody binding): 48 children 1–5 years of age who had relatives with celiac disease, enrolled nationwide; 31 healthy controls, children and young adults 2–18 years of age, enrolled at Massachusetts General Hospital (Appendix reference 17); and a population-based sample of 205 children and adults 3–50 years of age from a SARS-CoV-2 serosurvey in northern California, USA. We used the same method to generate individual-level incidence estimates of HlyE IgA and IgG responses and used the exponential probability distribution to calculate 2- and 4-year cumulative incidence. We then fit age-dependent curves by using generalized additive models (Appendix reference 18) with a cubic spline for age and simultaneous 95% CIs using a parametric bootstrap of the variance-covariance matrix of the fitted model parameters (Appendix reference 19).
A total of 2,214 persons were enrolled and provided blood samples for the original study; 1,840 had complete interview data, and 1,290 were randomly selected for testing (13). The median age of tested participants was 17 (interquartile range [IQR] 10–24) years; 63.5% (819/1,290) were female (Table 1).
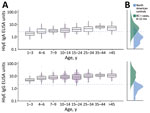
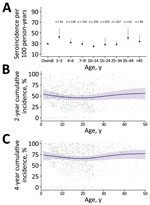
We found that median HlyE IgG (10.4, IQR 6.1–12.7) and IgA (3.5, IQR 2.3–5.2) responses were elevated well above a North America pediatric control population (IgG 0.16, IQR 0.07–0.35; IgA 0.3, IQR 0.001–0.92) and were comparable to responses observed among blood-culture confirmed enteric fever cases 8–12 months after symptom onset (IgG 12, IQR 5.9–24; IgA 4.4, IQR 2.2–9.4) (12) (Figure 1). Age-specific enteric fever incidence estimates per 100 person-years ranged from 24.8 (95% CI 23.6–28.8) among children 10–14 years of age to 42.5 (95% CI 38.0–59.0) among children 1–3 years of age (Table 2; Figure 2). The overall incidence rate was 29.8 (95% CI 27.6–32.2); cumulative incidence was 48.9% (IQR 31.9–64.3) over 2 years and 73.8% (IQR 53.7–87.3) over 4 years. Using a cutoff derived from a North America pediatric control population, we found 98.8% (1,275/1,290) of the population seropositive using HlyE IgG and 65.2% (318/488) positive using HlyE IgA (Appendix).
Using banked DBS collected for a SARS-CoV-2 serosurvey, we applied a new serosurveillance tool to rapidly estimate the burden of enteric fever in a region with no blood culture surveillance. We estimated an incidence rate of 30.0 infections/100 person-years and found >70% of the sampled population was infected in the previous 4 years.
Whereas no clinical enteric fever incidence estimates from South Sudan are available for comparison, the seroincidence rate we estimated is substantially higher than clinical incidence estimates in the region (15; Appendix reference 20). A high incidence of clinical enteric fever has been previously defined as >100 cases/100,000 person-years (Appendix reference 21); we estimated a seroincidence of 35,000 cases/100,000 person-years. We expect seroincidence to be higher than clinical incidence because it captures subclinical infections and is independent of a person’s ability to access and afford healthcare, including diagnostic tests. Indeed, the enteric fever seroincidence rate for Juba is on the same scale of magnitude as recent estimates using the same approach in Nepal, Pakistan, Bangladesh, and Ghana (12).
The analytic approach is an improvement over cutoff-based methods because we can combine information from HlyE IgA and IgG responses to generate a consensus incidence estimate, accounting for heterogeneity in antibody responses, measurement error, and biologic noise. Whereas the cutoff-based method yielded a seroprevalence of nearly 100% for HlyE IgG, we generated cumulative incidence estimates over a precise time window and could identify populations with recent and later infections.
Limitations of this study include that only 1,840 samples of 2,214 enrolled study participants had linked age data. Second, persons in internally displaced camps were not included in the serosurvey. Displaced persons have been identified as high-risk populations for enteric infections, so it would be valuable to include them in future studies to determine if this population is at higher or equivalent risk (4). Finally, we used longitudinal antibody kinetics estimates from enteric fever cases in Bangladesh, Pakistan, Nepal, and Ghana. We did not observe major differences in the kinetics of antibody responses across countries (12), but the decay rate among enteric fever cases in Juba may be different because of the high force of infection and differences in exposure to other infections.
Our results suggest a high burden of enteric fever in Juba, South Sudan, warranting urgent public health and research attention. The seroincidence tool we used can be applied to other regions lacking blood culture surveillance to generate rapid enteric fever seroincidence estimates, providing the high-resolution data critically needed to inform typhoid conjugate vaccine introduction.
Dr. Aiemjoy is an assistant professor of epidemiology at the University of California Davis School of Medicine. Her research centers on measurement, surveillance, and diagnostics for infectious diseases with a focus on seroepidemiologic methods to understand the force of infection in populations.
Acknowledgments
We gratefully acknowledge Julie Parsonnet, Catherine Ley, Alessio Fasano, Maureen M. Leonard, and Victoria Kenyon for generously sharing banked samples to define the distribution of antibody responses among individuals with no prior exposure to typhoidal Salmonella.
Accompanying code and de-identified data are available on Github (https://github.com/UCD-SERG/SSudanTyphoidSeroIncidence).
This work was supported by the World Health Organization’s Unity Studies, a global seroepidemiologic standardization initiative, with funding provided by the COVID-19 Solidarity Response. This study was also supported by The Bill and Melinda Gates Foundation (INV-000572) and the US National Institutes of Health (R01AI134814).
References
- Stanaway JD, Reiner RC, Blacker BF, Goldberg EM, Khalil IA, Troeger CE, et al.; GBD 2017 Typhoid and Paratyphoid Collaborators. The global burden of typhoid and paratyphoid fevers: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis. 2019;19:369–81. DOIPubMedGoogle Scholar
- Antillon M, Saad NJ, Baker S, Pollard AJ, Pitzer VE. The relationship between blood sample volume and diagnostic sensitivity of blood culture for typhoid and paratyphoid fever: a systematic review and meta-analysis. J Infect Dis. 2018;218(suppl_4):S255–67. DOIPubMedGoogle Scholar
- Voysey M, Pant D, Shakya M, Liu X, Colin-Jones R, Theiss-Nyland K, et al. Under-detection of blood culture-positive enteric fever cases: The impact of missing data and methods for adjusting incidence estimates. PLoS Negl Trop Dis. 2020;14:
e0007805 . DOIPubMedGoogle Scholar - Azman AS, Bouhenia M, Iyer AS, Rumunu J, Laku RL, Wamala JF, et al. High hepatitis E seroprevalence among displaced persons in South Sudan. Am J Trop Med Hyg. 2017;96:1296–301. DOIPubMedGoogle Scholar
- Abubakar A, Bwire G, Azman AS, Bouhenia M, Deng LL, Wamala JF, et al. Cholera epidemic in South Sudan and Uganda and need for international collaboration in cholera control. Emerg Infect Dis. 2018;24:883–7. DOIPubMedGoogle Scholar
- Kumar S, Nodoushani A, Khanam F, DeCruz AT, Lambotte P, Scott R, et al. Evaluation of a rapid point-of-care multiplex immunochromatographic assay for the diagnosis of enteric fever. MSphere. 2020;5:e00253–20. DOIPubMedGoogle Scholar
- Andrews JR, Khanam F, Rahman N, Hossain M, Bogoch II, Vaidya K, et al. Plasma immunoglobulin A responses against 2 Salmonella Typhi antigens identify patients with typhoid fever. Clin Infect Dis. 2019;68:949–55. DOIPubMedGoogle Scholar
- McClelland M, Sanderson KE, Clifton SW, Latreille P, Porwollik S, Sabo A, et al. Comparison of genome degradation in Paratyphi A and Typhi, human-restricted serovars of Salmonella enterica that cause typhoid. Nat Genet. 2004;36:1268–74. DOIPubMedGoogle Scholar
- Charles RC, Liang L, Khanam F, Sayeed MA, Hung C, Leung DT, et al. Immunoproteomic analysis of antibody in lymphocyte supernatant in patients with typhoid fever in Bangladesh. Clin Vaccine Immunol. 2014;21:280–5. DOIPubMedGoogle Scholar
- Charles RC, Sheikh A, Krastins B, Harris JB, Bhuiyan MS, LaRocque RC, et al. Characterization of anti-Salmonella enterica serotype Typhi antibody responses in bacteremic Bangladeshi patients by an immunoaffinity proteomics-based technology. Clin Vaccine Immunol. 2010;17:1188–95. DOIPubMedGoogle Scholar
- Charles RC, Sultana T, Alam MM, Yu Y, Wu-Freeman Y, Bufano MK, et al. Identification of immunogenic Salmonella enterica serotype Typhi antigens expressed in chronic biliary carriers of S. Typhi in Kathmandu, Nepal. PLoS Negl Trop Dis. 2013;7:
e2335 . DOIPubMedGoogle Scholar - Aiemjoy K, Seidman JC, Saha S, Munira SJ, Islam Sajib MS, Sium SMA, et al. Estimating typhoid incidence from community-based serosurveys: a multicohort study. Lancet Microbe. 2022;3:e578–87. DOIPubMedGoogle Scholar
- Wiens KE, Mawien PN, Rumunu J, Slater D, Jones FK, Moheed S, et al. Seroprevalence of severe acute respiratory syndrome coronavirus 2 IgG in Juba, South Sudan. Emerg Infect Dis. 2021;27:1598–606. DOIPubMedGoogle Scholar
- Garrett DO, Longley AT, Aiemjoy K, Yousafzai MT, Hemlock C, Yu AT, et al. Incidence of typhoid and paratyphoid fever in Bangladesh, Nepal, and Pakistan: results of the Surveillance for Enteric Fever in Asia Project. Lancet Glob Health. 2022;10:e978–88. DOIPubMedGoogle Scholar
- Marks F, von Kalckreuth V, Aaby P, Adu-Sarkodie Y, El Tayeb MA, Ali M, et al. Incidence of invasive salmonella disease in sub-Saharan Africa: a multicentre population-based surveillance study. Lancet Glob Health. 2017;5:e310–23. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 28, Number 11—November 2022
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Kristen Aiemjoy, University of California Davis, Public Health Sciences, 1 Shields Ave, Medical Sciences 1C, Davis, CA 95616-5270, USA
Top