Volume 28, Number 11—November 2022
Research
Differences in SARS-CoV-2 Clinical Manifestations and Disease Severity in Children and Adolescents by Infecting Variant
Figure 4
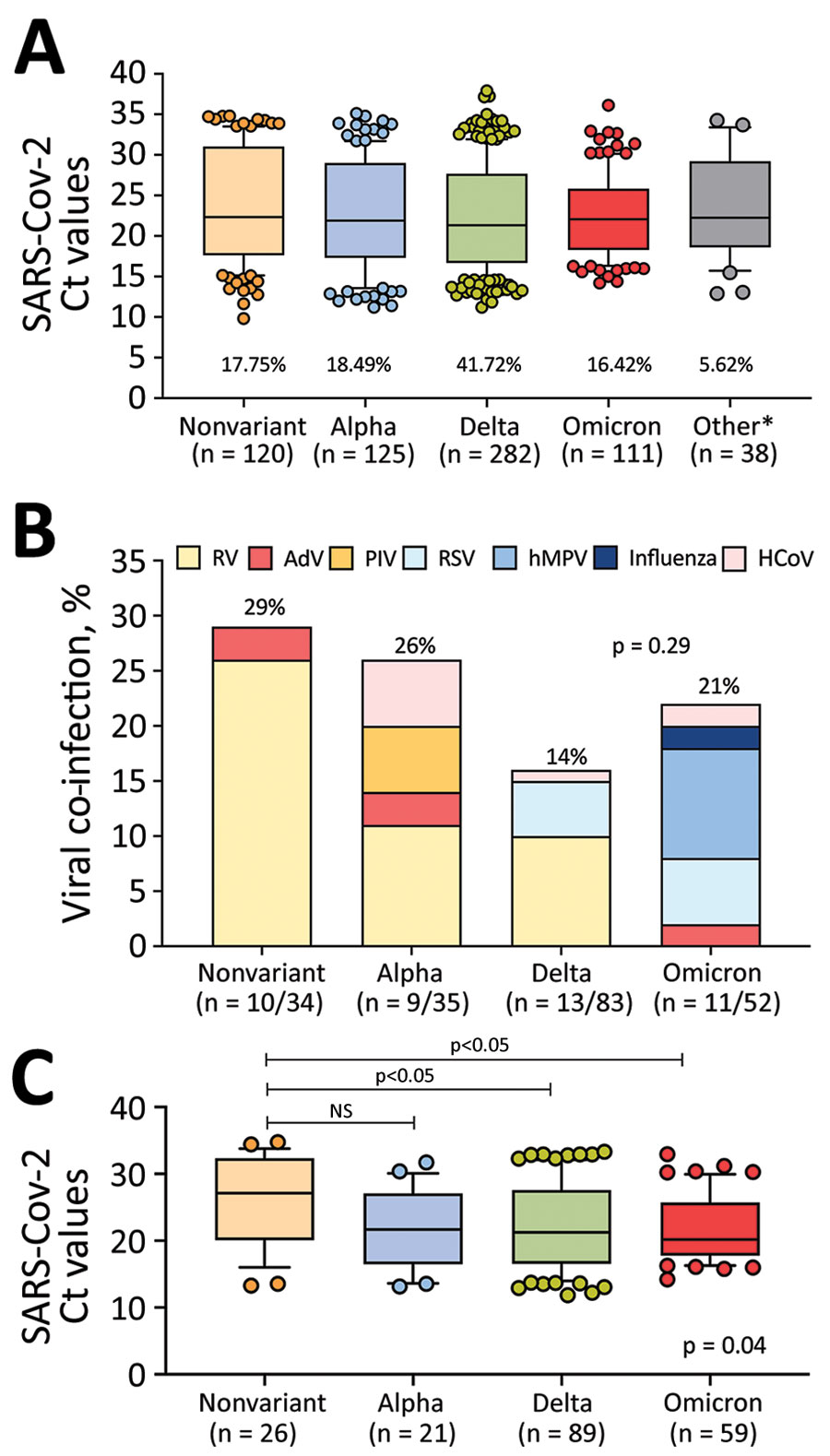
Figure 4. SARS-CoV-2 viral loads and viral co-infections among children and adolescents with COVID-19 at Nationwide Children’s Hospital, Columbus, Ohio, USA, by the infecting SARS-CoV-2 variant, January 1, 2021–January 15, 2022. A) Nasopharyngeal SARS-CoV-2 viral loads expressed as Ct values according to the infecting SARS-CoV-2 variant in the clinical cohort (n = 676). Percentage of total infections for each variant is below each bar. B) Viral co-infections by SARS-CoV-2 variant during the study period in patients that underwent multiplex viral testing. Twelve patients with other variants tested negative for viral co-infections (not shown). Percentage of total co-infections is above each bar. p value was determined by χ2 test. C) Nasopharyngeal SARS-CoV-2 Ct values by infecting SARS-CoV-2 variant among inpatients with acute COVID-19, excluding patients with MIS-C, SARS-CoV-2 detected by screening in inpatients, and those infected with uncommon SARS-CoV-2 strains. p value at bottom right represents the overall Kruskal-Wallis p value; values above bars indicate ad hoc pairwise comparisons by Dunn multiple test correction. For box plots in panels A and C, horizontal lines within boxes indicate medians; box tops and bottoms indicate interquartile ranges; error bars indicate 95% CIs. AdV, adenovirus; HCoV, human coronavirus; hPMV, human metapneumovirus; MIS-C, multisystem inflammatory syndrome in children; NS, not significant. PIV, parainfluenza virus; RSV, respiratory syncytial virus; RV, rhinovirus.
1These authors contributed equally to and co-directed this work.