Volume 28, Number 11—November 2022
Dispatch
Polyclonal Dissemination of OXA-232 Carbapenemase–Producing Klebsiella pneumoniae, France, 2013–2021
Figure 3
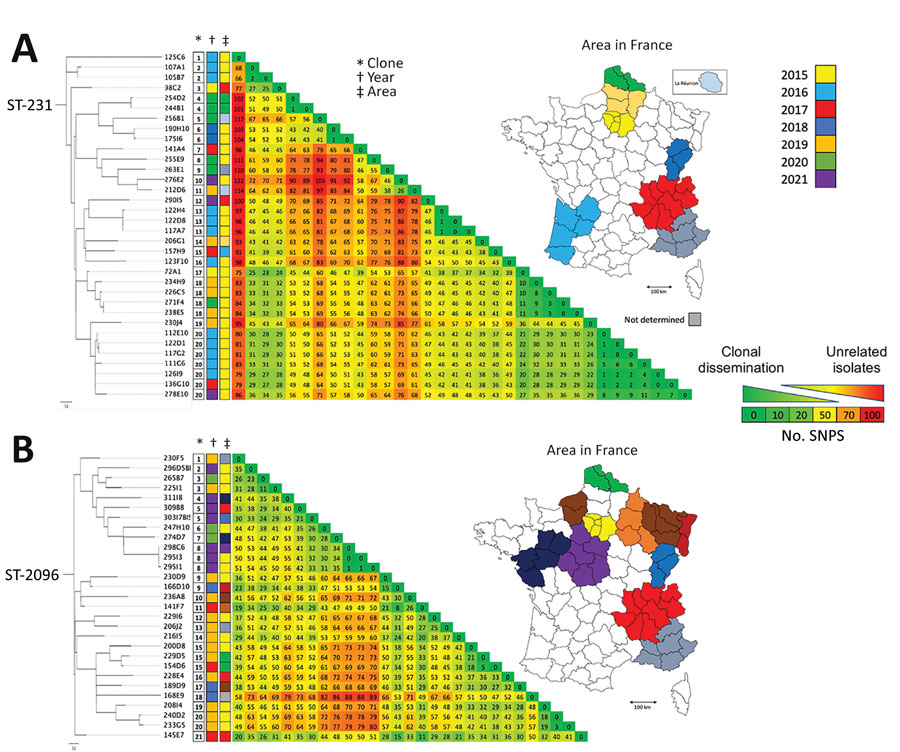
Figure 3. Global characterization (sequence type, year of isolation, β-lactamase content) of nonduplicate 95 OXA-232–producing Klebsiella pneumoniae analyzed at the National Reference Center for Carbapenem-Resistant Enterobacterales, France, 2013–2021. Scale bar indicates the number of SNP per position of common sequences. CMY-6, variant of C. freundii intrinsic cephalosporinase; CTX-M, cefotaximase–Munich extended-spectrum β-lactamase; OXA, oxacillinase; NDM, New Delhi metallo-β-lactamase; ST, sequence type, TEM, Temoniera β-lactamase.
Page created: August 17, 2022
Page updated: October 24, 2022
Page reviewed: October 24, 2022
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.