Volume 28, Number 12—December 2022
Research Letter
Laboratory Features of Trichinellosis and Eosinophilia Threshold for Testing, Nunavik, Quebec, Canada, 2009–2019
Cite This Article
Citation for Media
Abstract
Prolonged eosinophilia is characteristic of trichinellosis. To determine the optimal eosinophil threshold for reflex Trichinella testing, we examined all 43 cases in Nunavik, Quebec, Canada, during 2009–2019. Using receiver operating characteristic analysis, we determined that eosinophil counts >0.8 × 109 cells/L should prompt consideration of trichinellosis and testing to rapidly identify potential outbreaks.
Trichinella nativa infection is associated with ingestion of parasitized sylvatic animals and periodic outbreaks among residents of northern Canada (1–3). In the Arctic region of Nunavik in Quebec, outbreaks associated with polar bear and walrus consumption have prompted public health interventions, including a highly successful community-led active surveillance system that examines hunted meat for evidence of Trichinella encystment (4,5). We report a 10-year case series of Trichinella infection in Nunavik and describe the laboratory features. Eosinophilia is a well-characterized feature of infection that is readily available for most cases. We performed receiver operating characteristic (ROC) analysis to define an optimal threshold of eosinophilia to prompt reflex Trichinella antibody testing and rapid reporting to public health authorities for timely outbreak investigation (1–3).
In a retrospective test-negative case–control study, we reviewed laboratory and public health records to identify cases of trichinellosis in Nunavik that occurred from 2009 through 2019. Our study was approved by the Research Institute of the McGill University Health Centre Research and Ethics Board (REB #2020-5312).
We first reviewed all requests for Trichinella serologic testing sent from Quebec to the National Reference Centre for Parasitology, the only testing site for Quebec, during 2009–2019 (Appendix). To define an initial set of cases (with positive Trichinella serologic results), we selected specimens originating from Nunavik. One author (L.B.H.) reviewed the charts and confirmed cases if the clinical evolution was compatible with the positive serologic results. Because trichinellosis is notifiable by provincial law, we cross-referenced cases with the public health database to identify other cases determined epidemiologically and reviewed those charts. We defined a set of region-matched controls as those with negative Trichinella serologic results. Those controls are therefore persons from the general population from the same region who had clinical manifestations that prompted testing for trichinellosis. Although serologic results early in the disease course could be negative, chart review of controls did not yield additional suspected cases on the basis of clinical evolution. We extracted available clinical and laboratory data by chart review at the McGill University Health Centre and at regional health centers in Nunavik. We calculated summary statistics and tests (t-test and χ2), comparing cases and controls by using R (6), and generated ROC curves by using the pROC R package (7).
We identified 43 cases of trichinellosis and a set of 31 region-matched controls (Table). We excluded 4 possible case-patients with weakly positive serologic results but ambiguous clinical manifestations consistent with past infection. Information on signs and symptoms was available for only 19/43 case-patients, but demographic, laboratory, and clinical outcomes were well documented.
Case-patients had a median age of 40 years and were mostly female (30/43, 69.8%), which may result from chance (p = 0.052), differential exposure to parasitized meat, food sharing, or food preparation practices; or selection bias (8). When available, clinical features were similar to those previously described for trichinellosis (i.e., fever, rash and myalgia) (9). No patients died, and 9/27 (33%) patients with documented illness required hospitalization. Epidemiologic investigations revealed sporadic cases and 2 suspected point-source outbreaks (8). In 1 outbreak, seals were suspected as the source of infection, which could represent a change in epidemiology from previous outbreaks associated with polar bear and walrus meat and might reflect the surveillance program targeting game meat from the latter animals but not seals.
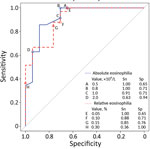
Laboratory information was available for 41/43 case-patients, a larger series of findings in Trichinella infection in Nunavik than previously reported. Features of Trichinella infection in Nunavik, presumptively caused by T. nativa, are similar to those reported for T. spiralis infection (9), including elevated creatinine kinase and eosinophilia (Table). The variable that differed most between cases and controls was peak absolute eosinophilia (5.35 vs. 0.80 × 109 cells/L; p<0.001). Among case-patients, peak eosinophilia was noted early and declined over months; among controls, counts were frequently elevated but stable over time (Appendix Figure). Using ROC analysis, we identified an absolute eosinophilia threshold of >0.8 × 109 cells/L, which identified all cases in this series with a specificity of 71% (Figure). We assessed the potential effect on resource use of this threshold by examining the region’s whole-population distribution of absolute eosinophil counts. Among 8,562 persons who submitted a specimen for complete blood count for any reason from January 2019 through April 2022, a total of 287 had eosinophil counts that exceeded our threshold (86 [1.2%] specimens/year).
Automated flags and reflex testing in Nunavik now incorporate the threshold identified in our analysis. In the absence of a defined alternative diagnosis, eosinophil counts of >0.80 ×109 cells/L should prompt clinical consideration of trichinellosis and further investigation. Early identification of outbreaks is critical in this region—where hunted meat is shared widely within and among communities—to limit exposures and enable delivery of postexposure prophylactic anthelmintic therapy, which has evidence of effectiveness in this serious illness (1,4,10). The cost–benefit ratio of this threshold will require ongoing assessment.
Dr. Harrison is chief resident in Infectious Diseases and Medical Microbiology at the McGill University Health Centre. His research interests include evolutionary biology.
Acknowledgments
We appreciate the collaboration of Nunavik Public Health, the National Reference Centre for Parasitology, and the staff of Inuulitsivik Health Centre, including members of medical records department.
This work was supported by a FRQ-S Clinician-Researcher Career Junior 2 award to C.P.Y.
References
- Schellenberg RS, Tan BJK, Irvine JD, Stockdale DR, Gajadhar AA, Serhir B, et al. An outbreak of trichinellosis due to consumption of bear meat infected with Trichinella nativa, in 2 northern Saskatchewan communities. J Infect Dis. 2003;188:835–43. DOIPubMedGoogle Scholar
- Dalcin D, Zarlenga DS, Larter NC, Hoberg E, Boucher DA, Merrifield S, et al. Trichinella nativa outbreak with rare thrombotic complications associated with meat from a black bear hunted in northern Ontario. Clin Infect Dis. 2017;64:1367–73. DOIPubMedGoogle Scholar
- MacLean JD, Viallet J, Law C, Staudt M. Trichinosis in the Canadian Arctic: report of five outbreaks and a new clinical syndrome. J Infect Dis. 1989;160:513–20. DOIPubMedGoogle Scholar
- Proulx JF, MacLean JD, Gyorkos TW, Leclair D, Richter AK, Serhir B, et al. Novel prevention program for trichinellosis in inuit communities. Clin Infect Dis. 2002;34:1508–14. DOIPubMedGoogle Scholar
- Larrat S, Simard M, Lair S, Bélanger D, Proulx J-F. From science to action and from action to science: the Nunavik Trichinellosis Prevention Program. Int J Circumpolar Health. 2012;71:18595. DOIPubMedGoogle Scholar
- R Core Team. R: a language and environment for statistical computing. Version 4.1.3 [cited 2022 Apr 1]. https://www.R-project.org
- Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. DOIPubMedGoogle Scholar
- Ducrocq J, Proulx J-F, Simard M, Lévesque B, Iqaluk M, Elijassiapik L, et al. The unique contribution of a local response group in the field investigation and management of a trichinellosis outbreak in Nunavik (Québec, Canada). Can J Public Health. 2020;111:31–9. DOIPubMedGoogle Scholar
- Gottstein B, Pozio E, Nöckler K. Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin Microbiol Rev. 2009;22:127–45. DOIPubMedGoogle Scholar
- Faber M, Schink S, Mayer-Scholl A, Ziesch C, Schönfelder R, Wichmann-Schauer H, et al. Outbreak of trichinellosis due to wild boar meat and evaluation of the effectiveness of post exposure prophylaxis, Germany, 2013. Clin Infect Dis. 2015;60:e98–104. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: November 18, 2022
Table of Contents – Volume 28, Number 12—December 2022
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Luke B. Harrison, McGill University Health Centre, Rm E5.1815, 1001 Blvd Decarie, Montreal, QC H4A 3J1, Canada
Top