Volume 28, Number 2—February 2022
Research Letter
Rickettsiosis Caused by Rickettsia parkeri, Mexico
Abstract
We report a human case of rickettsiosis caused by Rickettsia parkeri strain Atlantic Rainforest in Mexico in an adult woman from a small town in the north of Yucatan, Mexico. We confirmed diagnosis using conventional PCR and sequence analysis. Health providers should be aware of clinical manifestations of rickettsioses in this region.
Rickettsiosis caused by Rickettsia felis, R. rickettsii, and R. typhi is commonly reported in Mexico, mostly in the states of Sonora, Sinaloa, and Baja California (R. rickettsii) and Yucatan (R. felis, R. rickettsii, and R. typhi) (1). Rickettsiosis caused by R. parkeri (R. parketri) is a recently discovered disease. The first human case was reported in United States in 2004, and the bacterium was subsequently found in South America (2–5). R. parkeri infection is less virulent than infection with R. rickettsii, the agent of Rocky Mountain spotted fever. Signs and symptoms of R. parkeri infection are fever, rash, myalgia, and headache. Presence of eschar lesions at the inoculation site are common (5). In Mexico, R. parkeri strain Atlantic Rainforest has been identified in Amblyomma ovale ticks in Veracruz, R. parkeri sensu stricto in A. maculatum ticks collected from dogs in Sonora, and R. parkeri strain Black Gap in Dermacentor parumapertus ticks in Sonora and Chihuahua (6). These tick species are well distributed in Mexico; A. ovale ticks have been identified in northern, central, and southern areas and A. maculatum and D. parumapertus ticks in northern and central Mexico (7), suggesting that R. parkeri could be present in the entire country. We document a human case of R. parkeri rickettsiosis in the state of Yucatan.
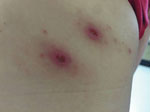
In January 2020, a 48-year-old woman from the municipality of Dzemul in northeastern Yucatan, where no previous cases of rickettsioses had been reported, went to a local health center because of fever (38°C) persisting for 2 days, myalgia, arthralgia in both legs, and abdominal pain. She had removed 2 ticks from her right upper back 2 days before seeking treatment (Figure). The patient stated that she had not traveled to other states or countries or to other regions within the Yucatan in the previous 2 months. Medics prescribed acyclovir and ibuprofen, but signs and symptoms persisted. The patient was therefore admitted to Hospital General Agustin O’Horan in Merida, the capital city of Yucatan. At the time of admission, the patient had fever (38°C), abdominal pain, diffuse arthralgias without rash, and left axillary lymphadenopathy. No palpable adenomegaly or hepatosplenomegaly were reported. We observed 2 skin lesions (12 × 15 mm and 11 × 16 mm, with erythematous haloes) at the bite site. The patient also reported itching and slight pain at the site of the tick bites (Figure).
Based on the tick bite and mild infection, the medic suspected rickettsiosis caused by R. felis. For conventional PCR diagnosis, we obtained a 3-mL whole blood sample and sent it to our laboratory. In regions where rickettsiosis caused by R. parkeri are common, medics usually take samples from eschar lesions (5,8,9). We obtained a skin biopsy from the eschar lesion 2 days after treatment was initiated. However, conventional PCR analysis returned negative results.
We purified DNA from the blood sample following the instructions from a Quick-gDNA MiniPrep Kit (Zymo Research, https://www.zymoresearch.com) and used conventional PCR to amplify 2 gene fragments (gltA and ompB) for identifying Rickettsia spp. Primer sequences and amplification conditions are described by the Latinamerican Guidelines of RIICER for Diagnosis of Tick-Borne Rickettsiosis (10). For the gltA gene, we amplified a 380-bp fragment and for the ompB gene, a 420-bp fragment. We used sterile water as the negative control and R. typhi DNA as the positive control. We purified and sequenced products from the PCR. We analyzed DNA sequences using blastn (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and found the amplified products (GenBank accession nos. MW653956 and MW653957) 100% homologous to R. parkeri strain Atlantic Rainforest from human cases in Brazil (GenBank accession no. MN027564.1) (8) and Colombia (GenBank accession no. MK860201.1) (5), as well as to a recently identified R. parkeri isolate (GenBank accession no. MK814825.1) from A. ovale in Veracruz, Mexico. After diagnosing the Rickettsia spp. infection, we treated the patient with doxycycline (100 mg 2×/d for 10 d). After day 2, fever and other symptoms ceased.
Our study documents a case of human rickettsiosis caused by R. parkeri strain Atlantic Rainforest in Yucatan, Mexico. This finding represents the fifth Rickettsia species identified as infecting humans in southeastern Mexico, but in a municipality, Dzemul, with no previous Rickettsia spp. infections reported among humans or identified in vectors or reservoir hosts. Because this rickettsiosis causes mild to moderate febrile illness with initial symptoms such as fever, headache, muscle pain, nausea, vomiting, rash (2), it might masquerade as dengue fever in our region and other areas where dengue is common.
Our report emphasizes the importance of continuing to characterize clinical manifestations of rickettsioses in Mexico. Health providers in this region should include this recently discovered rickettsiosis in differential diagnoses of febrile illnesses.
Dr. Peniche-Lara works at Facultad de Medicina at Universidad Autonoma de Yucatán, Mexico. His research focuses on the epidemiology of pathogens transmitted by ticks in southeastern Mexico.
Acknowledgment
Dedicated to the late Víctor Manuel Lara-Perera for his clinical and critical medical eye for the identification of rickettsial diseases and great commitment in teaching and medical practice. We will miss you forever.
References
- Sánchez-Montes S, Colunga-Salas P, Lozano-Sardaneta YN, Zazueta-Islas HM, Ballados-González GG, Salceda-Sánchez B, et al. The genus Rickettsia in Mexico: Current knowledge and perspectives. Ticks Tick Borne Dis. 2021;12:
101633 . DOIPubMedGoogle Scholar - Paddock CD, Sumner JW, Comer JA, Zaki SR, Goldsmith CS, Goddard J, et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805–11. DOIPubMedGoogle Scholar
- Pacheco RC, Venzal JM, Richtzenhain LJ, Labruna MB. Rickettsia parkeri in Uruguay. Emerg Infect Dis. 2006;12:1804–5. DOIPubMedGoogle Scholar
- Silveira I, Pacheco RC, Szabó MP, Ramos HG, Labruna MB. Rickettsia parkeri in Brazil. Emerg Infect Dis. 2007;13:1111–3. DOIPubMedGoogle Scholar
- Arboleda M, Acevedo-Gutiérrez LY, Ávila A, Ospina D, Díaz FJ, Walker DH, et al. Human rickettsiosis caused by Rickettsia parkeri strain Atlantic Rainforest, Urabá, Colombia. Emerg Infect Dis. 2020;26:3048–50. DOIPubMedGoogle Scholar
- Sánchez-Montes S, Ballados-González GG, Hernández-Velasco A, Zazueta-Islas HM, Solis-Cortés M, Miranda-Ortiz H, et al. Molecular confirmation of Rickettsia parkeri in Amblyomma ovale ticks, Veracruz, Mexico. Emerg Infect Dis. 2019;25:2315–7. DOIPubMedGoogle Scholar
- Guzmán Cornejo C, Robbins RG, Guglielmone AA, Montiel-Parra G, Pérez TM. The Amblyomma (Acari: Ixodida: Ixodidae) of Mexico: identification keys, distribution and hosts. Zootaxa. 2011;2998:16–38.
- da Paixão Sevá A, Martins TF, Muñoz-Leal S, Rodrigues AC, Pinter A, Luz HR, et al. A human case of spotted fever caused by Rickettsia parkeri strain Atlantic rainforest and its association to the tick Amblyomma ovale. Parasit Vectors. 2019;12:471. DOIPubMedGoogle Scholar
- Silva-Ramos CR, Hidalgo M, Faccini-Martínez ÁA. Clinical, epidemiological, and laboratory features of Rickettsia parkeri rickettsiosis: A systematic review. Ticks Tick Borne Dis. 2021;12:
101734 . DOIPubMedGoogle Scholar - Oteo JA, Nava S, Sousa R, Mattar S, Venzal JM, Abarca K, et al.; RIICER. [Latinamerican guidelines of RIICER for diagnosis of tick-borne rickettsioses] [in Spanish]. Rev Chilena Infectol. 2014;31:54–65. DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: January 13, 2022
1These authors contributed equally to this article.
Table of Contents – Volume 28, Number 2—February 2022
| EID Search Options |
|---|
|
|
|
|
|
|