Volume 28, Number 3—March 2022
Research
High-Dose Convalescent Plasma for Treatment of Severe COVID-19
Figure 3
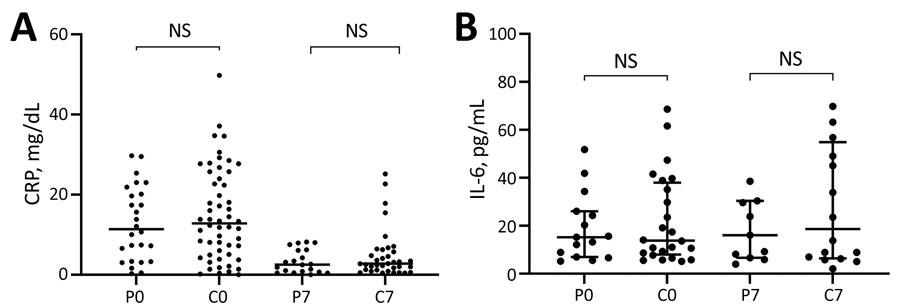
Figure 3. Scatter plots of inflammatory biomarker levels among participants in study of high-dose convalescent plasma for treatment of severe COVID-19, Brazil. A) C-reactive protein (CRP); total 80 patients (26 CCP, 54 control) on day 0 and 56 (20 CCP, 36 control) on day 7. B) Interleukin-6 (IL-6); total 39 patients (15 CCP, 24 control) on day 0 and 27 (11 CCP, 16 control) on day 7. Horizontal bars indicate medians. C0, control group day 0; C7, control group day 7; COVID-19, coronavirus disease; CCP, COVID-19 convalescent plasma; NS, not significant; P0, convalescent plasma group day 0; P7, convalescent plasma group day 7.
Page created: January 26, 2022
Page updated: February 21, 2022
Page reviewed: February 21, 2022
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.