Volume 28, Number 6—June 2022
Research Letter
Experimental Infection of Mink with SARS-COV-2 Omicron Variant and Subsequent Clinical Disease
Figure
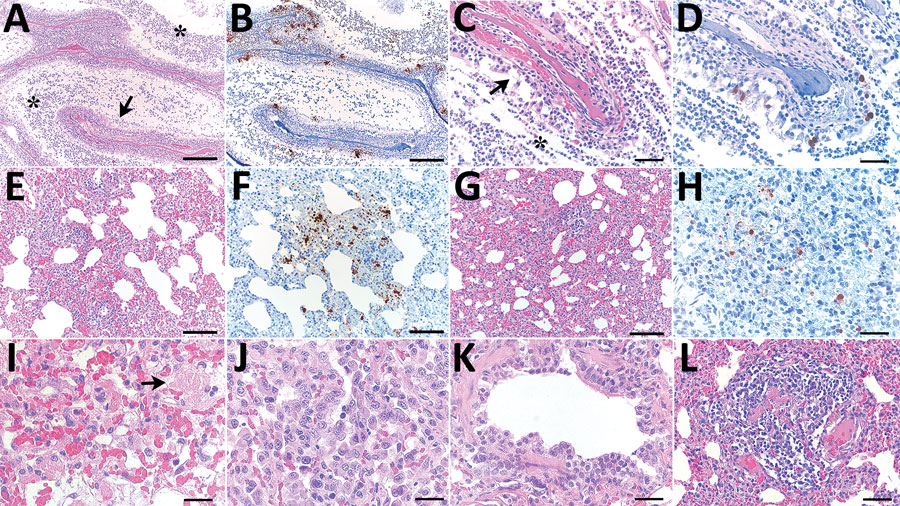
Figure. Histopathologic changes and SARS-CoV-2 expression in the upper and lower respiratory tracts of mink experimentally infected with Omicron variant at 7 days postinfection and recipient mink after 10 days of follow-up. A) Respiratory segment of the nose from an intranasally infected mink showing luminal accumulation of exudate (asterisks) and degeneration of mucosal epithelium (arrow) Scale bar indicates 500 µm. B) Viral antigen widely detected within nasal lumen and respiratory epithelium. Scale bar indicates 500 µm. C, D) Respiratory epithelium from a recipient mink depicting marked degeneration and loss (arrow in panel C) and intraluminal accumulation of sloughed cells and neutrophils (asterisk in pane C), and intraepithelial viral expression (panel D). Scale bars indicate 50 µm. E–H) Lungs from intranasally infected (E, F) and recipient (G, H) mink showing alveolar damage with intralesional presence of viral nucleoprotein. Scale bars indicate 200 µm in panels E–G and 50 µm in panel H. I, J) Marked degeneration and necrosis of alveolar septa and focal hyalin membrane (arrow in panel I) and prominent proliferation of type II pneumocytes (panel J) in an intranasally infected mink. Scale bars in indicate 25 µm). K, L) Recipient mink showing bronchiolar epithelial degeneration and hyperplasia (K) and vasculitis (L) with complete destruction of blood vessel wall and mononuclear cell infiltration. Scale bar indicates 50 µm in panel K and 100 µm in panel L. Hematoxylin and esosin stain and immunohistochemistry, hematoxylin counterstain.
1These authors contributed equally to this article.