Volume 28, Number 6—June 2022
Research
Risk Factors for SARS-CoV-2 Infection and Illness in Cats and Dogs1
Abstract
We tested swab specimens from pets in households in Ontario, Canada, with human COVID-19 cases by quantitative PCR for SARS-CoV-2 and surveyed pet owners for risk factors associated with infection and seropositivity. We tested serum samples for spike protein IgG and IgM in household pets and also in animals from shelters and low-cost neuter clinics. Among household pets, 2% (1/49) of swab specimens from dogs and 7.7% (5/65) from cats were PCR positive, but 41% of dog serum samples and 52% of cat serum samples were positive for SARS-CoV-2 IgG or IgM. The likelihood of SARS-CoV-2 seropositivity in pet samples was higher for cats but not dogs that slept on owners’ beds and for dogs and cats that contracted a new illness. Seropositivity in neuter-clinic samples was 16% (35/221); in shelter samples, 9.3% (7/75). Our findings indicate a high likelihood for pets in households of humans with COVID-19 to seroconvert and become ill.
SARS-CoV-2 originated in horseshoe bats and probably reached humans through an unidentified intermediary host (1). The virus is aerosolized and highly transmissible among humans; new variants have arisen and spread in successive waves across the world since late 2019. Since a report of SARS-CoV-2 infection in a dog in March 2020 (2), an ever-increasing range of species has been shown to be susceptible to infection, including household cats, dogs, ferrets, and hamsters (3–10).
Companion animals have closest contact with humans, creating ample opportunity for exposure. Experimental infections have suggested that most companion animals are infected only transiently, as indicated by PCR positivity or virus isolation (11,12). Conversely, detection of antibodies by ELISA or neutralizing antibody assay suggests infection rates of 0.2%–43.9% related to factors such as the likelihood and frequency of interaction with infected humans (13–16). Infections in animals are typically subclinical or associated with transient respiratory or gastrointestinal disease (17,18). In rare cases, death has been attributed to SARS-CoV-2 infection; however, defining the contribution of SARS-CoV-2 to death in animals with underlying conditions such as cancer, bacterial pneumonia, or obesity is challenging. On the other hand, minks are highly susceptible to infection and pneumonia, and mortality rates of 35%–55% caused by SARS-CoV-2 infection were reported from outbreaks among farmed mink in Utah (19). Captive minks also contracted viruses with a unique amino acid substitution in the spike (S) protein that were subsequently retransmitted to humans and to community cats and dogs, around mink farms in the Netherlands (5,20). Similarly, infected pet Syrian hamsters may also retransmit SARS-CoV-2 to humans (21). More than 30% of free-ranging white-tailed deer tested in Ohio were SARS-CoV-2 positive by PCR, and a similarly high proportion of white-tailed deer in Texas and other North America locations had neutralizing antibodies (22,23). Experimentally, white-tailed deer transmitted SARS-CoV-2 to other deer vertically and horizontally by direct contact (24). It has not yet been determined if infected deer experience illness or have increased illness and death rates or if transmission is sustained among wild deer populations. However, such high prevalence suggests SARS-CoV-2 may become endemic in some deer populations in North America.
SARS-CoV-2 is transmitted predominantly via aerosols, aided by proximity of infected and susceptible hosts, the degree of host susceptibility, and the concentration of infectious virions in air. Although most infections in animals originate from humans, neither risk factors for zoonotic transmission from humans to pets nor the frequency and nature of clinical illness in pets are well defined. We report the frequency of SARS-CoV-2 seropositivity in cohorts of pets from households, low-cost neuter clinics, and animal shelters in Ontario, Canada, and analyze household risk factors associated with seropositivity. The University of Guelph (Ontario, Canada) approved the studies by Animal Utilization Protocol 4411 and Research Ethics Board Protocol 20-04-002.
Swab Samples
Pet owners who had a diagnosis of SARS-CoV-2 infection or symptoms compatible with COVID-19 in the previous 3 weeks were invited to have their pet swabbed by study veterinarians during April 24, 2020–August 31, 2021. Dogs, cats, and ferrets of any age and clinical status were eligible for testing; the only exclusion criterion was medical or behavioral issues that precluded safe sampling. We obtained swab samples from the distal nares, oropharynx, and rectum, whenever possible. We placed swabs in inactivating media (PrimeStore; Longhorn Vaccines and Diagnostics, https://lhnvd.com) for a minimum of 12 hours, extracted RNA using Galvens Viral RNA Extraction (Montreal Biotech, https://www.montreal-biotech.com), and eluted into water.
We performed quantitative reverse transcription PCR to amplify SARS-CoV-2 cDNA with primers and probe in the viral N1 gene (Appendix 1). We submitted samples with positive results for amplification of segments of the envelope (E) and RNA-dependent RNA polymerase (RdRp) genes and whole-genome sequencing in additional laboratories.
Serum Samples
During June 8, 2020–November 30, 2021, we invited owners of pets who received a diagnosis of SARS-CoV-2 infection 2 weeks–3 months previously to have a blood sample of their pet analyzed for SARS-CoV-2 antibodies.
Veterinarians or veterinary technicians at Toronto Humane Society (THS) collected blood samples from cats and dogs admitted to the shelter during June 18–November 28, 2020. Any animal that did not have health and behavioral reasons for exclusion was eligible for the study, regardless of origin (surrender, seizure, stray) or known history of SARS-CoV-2 exposure. Similarly, we collected samples through Toronto Animal Services (TAS) from unowned and owned cats admitted to a low-cost neuter clinic during January 21–July 6, 2021. We centrifuged all blood samples on site and shipped serum samples to Ontario Veterinary College (Guelph, Ontario, Canada). Serum samples were frozen in aliquots until batch analysis.
We constructed ELISA assays for the detection of cat and dog IgG and IgM to SARS-CoV-2 S protein (Appendix 1). Positive controls were from a SARS-CoV-2–experimentally-infected cat and 2 dogs with high titers; negative controls were cat and dog serum samples collected before 2019.
We tested the initial 42 serum samples and a subsequent 70 samples with IgG optical density (OD) >1.4 with the surrogate virus neutralization test (sVNT; GenScript, https://www.genscript.com) to determine blocking of the interaction of the receptor-binding domain (RBD) of SARS-CoV-2 with the ACE2 receptor. Following manufacturer instructions, we interpreted inhibition >20% relative to the kit positive control as indicating the presence of neutralizing antibodies.
Survey
We asked owners of household pets to complete an online 20-question survey concerning household demographics, the nature of the interaction with their pets, and the development of new illness in pets (Appendix 2). We also administered a questionnaire to owners of cats brought to the low-cost neuter clinic (Appendix 3). Questionnaires were not administered for unowned cats.
Statistical Analysis
For household cats, factors associated with PCR positivity were not evaluated because of the small sample size and low prevalence. We evaluated factors associated with seropositivity by univariable analysis using χ2, Fisher exact, or Wilcoxon tests as appropriate for the data. We categorized neuter-clinic cats by age: cats <6 months of age were kittens and cats >6 months adults. We calculated odds ratios and 95% CI. We did not perform multivariable analysis because of limitations in sample size.
We compared differences in seropositivity among different pet cohorts with Mann-Whitney tests. We calculated correlation of ELISA OD with sVNT results using Prism version 9.3.1 (GraphPad, https://www.graphpad.com); p<0.05 was considered significant.
PCR
We collected a total of 283 swab specimens from 65 cats, 49 dogs, and 6 ferrets: 70 nasal, 90 oral, 107 rectal and 16 fur (dorsum) samples. Samples from 5 (7.7%) cats and 1 (2.0%) dog had positive PCR results. Each N1 PCR positive result (Ct <35.99) was confirmed by amplification with E, R, or RdRp primers. For all 6 animals testing positive, the nasal swab samples were positive; oral swab samples were positive from 2 of 3 tested, and rectal swab samples were positive from 1 of 3 tested. Swab samples from an additional 10 (15%) cats, 3 (6.1%) dogs, and 3 (50%) ferrets had nonnegative results. N1 PCR Ct values for those 16 samples were 36.00–39.00. Testing of other targeted regions at additional laboratories yielded similar nonnegative results.
One cat with an initial Ct of 21.56 was retested weekly 5 times after the first positive result and had positive results during the first 3 weeks. Another cat with an initial Ct of 24.11 tested positive again 1 week later (Ct 36.19) and negative thereafter.
We derived whole-genome sequences (>99.3% coverage) from 2 positive cats. Phylogenetic analysis assigned the sequences to Pangolin lineage A.23.1 and B.1.2, which had the highest similarity to human SARS-CoV-2 sequences derived in that time period from the corresponding geographic region.
Serology
Household Pets
We collected serum samples from 59 dogs and 48 cats from 77 households and 1 animal shelter (from recently surrendered cats). Median number of samples per household was 1 (range 1–4). We collected 7 samples from the humane society; those 7 samples were excluded from risk factor analysis because of the potential clustering effect and the lack of metadata about these animals. Dogs were a median of 5 years of age (range 5 months–14 years of age), and cats were a median of 6 years of age (range 1–19 years of age).
Seropositivity for IgG and IgM was 42%–62% using >3 SD above the mean of the negative control samples as a cutoff and 25%–48% at >6 SD (Table 1). At >6 SD, all IgM positive dogs were also IgG positive, whereas 12/48 (25%) cats were IgG positive but IgM negative.
For statistical analysis, we defined seropositivity as >3 SD for IgG, IgM, or both. We observed a significant association between seropositivity and owner-reported onset of new respiratory disease in dogs at the time of the owner’s infection (p = 0.04), but not in cats (Table 2). Association of seropositivity and owner-reported new onset of clinical signs (respiratory, gastrointestinal, or systemic signs such as lethargy) approached significance in dogs (p = 0.06).
Not all risk factor data were available for all animals. Univariable risk factor analysis did not identify risk factors for dogs, but sleeping in the owner’s bed was a risk factor for seropositivity in cats (OR 5.8, 95% CI 1.1–29.4) We determined no effect from the presence of multiple pets in the household (dogs p = 0.33, cats p = 0.70) or the number of persons with confirmed (dogs p = 0.77, cats p = 0.64) or confirmed and suspected (dogs p = 0.92, cats p = 0.47) COVID-19. We did not see an association between time the animal typically spent per day with the infected owner for either dogs (p = 0.71) or cats (p = 0.53).
When we defined seropositivity as >6 SD above the mean of negative controls, we saw no significant association between seropositivity and owner-reported onset of new respiratory disease in the pet at the time of the owner’s infection for dogs (Table 3). However, we observed a significant association of seropositivity and owner-reported new onset of clinical respiratory, gastrointestinal, or systemic signs such as lethargy in the pet. We found the same association in cats.
Univariable risk factor analysis did not identify an association of seropositivity with risk factors (Table 3). We saw no association between time the animal typically spent per day with the infected owner for either dogs (p = 0.73) or cats (p = 0.35). However, cats that spent <2 hours per day with their owner were significantly less likely to be seropositive (1/7 [16%] vs. 18/30 [67%]; p = 0.04). We did not see the same result for dogs (p = 0.51). We saw no effect from the presence of multiple pets in the household (dogs p = 0.61, cats p = 0.69) or the number of persons per household with confirmed (dogs p = 0.83, cats p = 0.74) or confirmed or suspected (dogs p = 0.84, cats p = 0.82) COVID-19. Overall, >1 animal was seropositive in 3 (16%) of the 19 households where >1 animal was sampled: 2 households in which 2 dogs were seropositive and 1 in which a dog and cat were seropositive.
We performed sVNT on 53 samples from household pets. Of those, 30/41 (76%) that were positive for IgG and/or IgM (6 SD) were also positive on sVNT compared with 0/12 IgG/IgM negative samples (p<0.0001). Despite the smaller sample size, we repeated risk factor analysis using the samples tested by sVNT. For dogs, licking hands or face of owners was associated with seropositivity (OR 10.5 95% CI 1.5–73; p = 0.017). In addition, we noticed an association between positivity and dogs spending 19–24 hours with owners (OR 13.3, 95% CI 1.3–135; p = 0.033). For cats, the association between positivity and being kissed by owners was significant (OR 18.7, 95% CI 1.6–223; p = 0.020).
Neuter-Clinic Cats
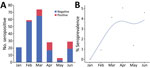
We collected serum samples from 221 cats during January–June 2021. Full animal information and history were not available for all cats. The median age of the 184 (83%) cats for which age was reported or estimated was 1.5 years (interquartile range 3.25 years). We classified 32/184 (17%) cats as kittens and 152 (83%) as adults (Table 4). COVID contact status was known for 103 cats. We detected S IgG (>6 SD) in 35/221 (16%) cats. Monthly seropositivity rate was 0%–40%; we identified a significant association between month and seropositivity (p<0.0001) (Figure 1).
Univariable analyses were performed excluding animals whose exposure to persons with COVID-19 was unknown (Table 4). We identified animal source as a risk factor for seropositivity. Compared with cats originating from households, cats that were in a shelter, rescue or foster facility cats were 3.6 times as likely to be seropositive (95% CI 1.5–8.8; p = 0.005). We found no significant difference between feral and household cats or feral and shelter/rescue/foster cats.
Humane Society Animals
Of 67 cat and 8 dog samples from THS, 7/75 (9.3%) overall and 7/67 (10%) of cat samples were seropositive (>6 SD). We did not perform risk factor analysis because limited metadata were available.
Correlation of ELISA with sVNT
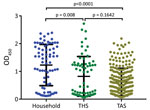
We identified a significant difference in the mean OD between household samples and those from both THS and TAS. Differences between THS and TAS were not significant (Figure 2).
In addition to ELISA testing, we also assessed a subset of 112 serum samples (53 household and 59 from shelter and spay/neuter clinic) with the sVNT. We found a significant correlation between the ELISA OD and neutralization of virus binding (ρ = 0.4188, 95% CI 0.2529–0.5608; p<0.0001) (Figure 3, panel A). The correlation between ELISA and sVNT results was higher for cats than dogs (Figure 3, panel B).
Our findings suggest that transmission of SARS-CoV-2 from infected humans to their pets as indicated by seroconversion is common. PCR-based detection of SARS-CoV-2 in pets was uncommon within 3 weeks from owners being symptomatic or having a diagnosis of COVID-19, which may reflect genuine brevity of infection in pets, as noted experimentally in cats (12).Other factors are variations in time intervals between owner infection and pet sampling and the challenge of obtaining representative samples from the nose in cats (12). Other studies of infection of cats from households of persons with COVID-19 had similarly low PCR-based prevalence (16,25–28). The timeframe required for owners to be diagnosed, contact the study team, and arrange a household visit likely resulted in false negative PCR results from samples being collected too late relative to onset of infection. The definition of COVID-19 symptoms and access to PCR testing for sick persons was limited early in the pandemic, and it is possible that pets in this study were infected concurrently or immediately after their owners but swabbed only after they had eliminated infection. Kittens 4–5 months old experimentally infected with 1 × 106 TCID50 of SARS-CoV-2 intranasally and orally had detectable viral RNA for 10 days in nasopharyngeal swabs, 7 days in oropharyngeal swabs, and 14 days in rectal swabs, but such high viral challenge may not simulate typical human–cat household interactions (12). Subtle pulmonary lesions and viral RNA detectable until 6 days postinfection in experimentally infected cats suggest that, even with high viral inoculates, cats rarely get sick and can clear infection relatively quickly (29).
Longitudinal samples were rarely available; however, serial sampling for 1 cat revealed prolonged PCR positivity. That cat had chronic upper respiratory disease; whether the condition played a role in the prolonged PCR positivity is unclear. Despite the duration of PCR positivity, it is unlikely that the cat was infectious because the relatively high PCR Ct values would be consistent with low-level shedding of viral nucleic acids. Similar prolonged PCR positivity has been reported for a cat exposed in a retirement home (30) and for tigers and lions in zoos (31). More data regarding the duration of positivity in naturally infected dogs and cats, and whether infectious virus is shed, are needed.
Seroprevalence was much higher than PCR positivity. We expected this finding because serologic data represent historical exposure and there is not a need to sample animals within a narrow infection window. Seroprevalence detected in other studies was 0.4%–30% or higher; in most instances such variability could be attributed to the extent of pets’ exposure to infected humans (6,9,32–34).
Without broadly accepted definitions, the parameters and interpretation of serologic assays for SARS-CoV-2 vary widely (13,28,35–37). We designed traditional ELISAs detecting IgG and IgM for S protein. We used a range of negative serum samples from before 2019, as well as serum from cats with feline infectious peritonitis caused by enteric α coronavirus. The negative controls yielded consistently low ODs for S protein IgG and IgM; we interpreted results from exposed animals at 3 SD and 6 SD above the mean of the least diluted negative controls to enable comparison with other studies (12,13). A relatively high proportion of dogs and cats had antibodies to S protein, which could indicate infection or exposure. Results of the sVNT, most likely to reflect infection, correlated with S protein ELISA results in this and other studies (38). Some serum samples had high S antibodies despite lack of neutralization; this pattern could indicate exposure rather than infection or postinfection persistence of antibodies broadly reactive with S protein but not neutralizing RBD binding. The cause of the discrepant results cannot be determined from samples collected at a single time point that was potentially days or weeks postexposure. Furthermore, development of antibodies to SARS-CoV-2 is affected by host age, immunocompetence, and comorbidities, which could not be controlled in this surveillance study (36); even experimentally infected young cats had inconsistent antibody responses (12).
Risk factor analyses identified plausible associations presumably linked to the duration and closeness of human–animal contact. Limited risk factor information for dogs and cats has been reported (16, 28,37,39); however, association of seropositivity and proximity or sleeping with infected owners has been reported for dogs (16) and in a study where canine and feline data were combined (40). In our study, the same risk factors were not identified when using different serologic cutoffs or tests, which was likely a result of small sample sizes.
The substantially higher seroprevalence in cats exposed to infected persons gives more credence to the seropositivity data. Yet, the prevalence of seropositivity was still moderately high in cats with no known exposure to infected people. The lack of metadata makes this challenging to interpret, because it is possible that cats from the humane society or neuter clinic had previously been exposed to infected humans (28).
PCR positivity rate was too low for robust comparison of sample sites. However, all positive animals had positive nasal swab specimens, despite the challenges that can be encountered collecting good nasal swabs, especially from cats. Adding oral, rectal, or fur swab specimens did not increase diagnostic yield. Further study of sampling sites under field conditions would identify sampling approaches that maximize diagnostic yield while minimizing the number of sites that must be sampled. These data are preliminary but support the importance of collecting nasal swab specimens as part of or all of the sample set.
Our study’s first limitation was sample size; enrollment was hampered by low human COVID-19 infection rates in the study region throughout the main sampling times and by difficulties identifying exposed households in an appropriate timeframe. Lack of a coordinated One Health approach concurrently investigating human and animal exposures was a problem; local or provincial public health agencies had little interest in leading research or performing a joint study. The timing of sampling also affected PCR results. More complete validation of the specificity of serologic assays with a samples from animals with diverse other infectious and inflammatory conditions remains to be done. Ideally, the timeframe for sampling would have been more condensed to focus testing on animals whose owners were more recently infected (e.g., 1–2 weeks after the onset of the owner’s infection).
These data indicate relatively common transmission of SARS-CoV-2 from humans to animals and that certain human–animal contacts (e.g., kissing the pet, pet sleeping on the bed) appear to increase the risk. We inferred that infections in dogs and cats reflect direct transmission from humans to animals, given the pandemic nature of this virus in humans and limited contact of most household pets with other animals (41). Intra-household transmission cannot be ruled out as a cause of some infections; however, multiple seropositive animals were only identified in 3/19 (16%) households where multiple animals were tested. We did not specifically investigate whether this relates to differences in individual animal susceptibility or animal–owner contact.
The relevance of human–pet transmission of SARS-CoV-2 needs further study. We observed an association between infection and clinical disease in both dogs and cats; in most cases, disease was very mild and self-limiting. Clinical data from this study are consistent with other studies indicating limited overall health risk to otherwise healthy dogs and cats (17,18,42). The zoonotic risk posed by dogs is probably low based on the lower infection rate and lack of evidence of transmission experimentally (43). Risk is probably higher for cats; cat–cat transmission has been identified, but the actual risk for cat–human transmission is unknown (44). Our findings support the occurrence of human–dog and human–cat transmission and highlight the need for further study of the animal and human health consequences of spillback of this zoonotic pathogen into animals.
Dr. Bienzle is a professor of veterinary pathology at Ontario Veterinary College. Her research interests include infectious diseases of companion animals.
Acknowledgments
We thank the animal owners who provided samples and information for this study, and the veterinarians and veterinary technicians who assisted with sample procurement.
Funding was provided by the Ontario Ministry of Agriculture via the Ontario Animal Health Network and by the University of Guelph.
References
- Koopmans M, Daszak P, Dedkov VG, Dwyer DE, Farag E, Fischer TK, et al. Origins of SARS-CoV-2: window is closing for key scientific studies. Nature. 2021;596:482–5. DOIPubMedGoogle Scholar
- Almendros A, Gascoigne E. Can companion animals become infected with Covid-19? Vet Rec. 2020;186:419–20. DOIPubMedGoogle Scholar
- Bosco-Lauth AM, Root JJ, Porter SM, Walker AE, Guilbert L, Hawvermale D, et al. Peridomestic mammal susceptibility to severe acute respiratory syndrome coronavirus 2 infection. Emerg Infect Dis. 2021;27:2073–80. DOIPubMedGoogle Scholar
- Decaro N, Vaccari G, Lorusso A, Lorusso E, De Sabato L, Patterson EI, et al. Possible human-to-dog transmission of SARS-CoV-2, Italy, 2020. Emerg Infect Dis. 2021;27:1981–4. DOIPubMedGoogle Scholar
- van Aart AE, Velkers FC, Fischer EAJ, Broens EM, Egberink H, Zhao S, et al. SARS-CoV-2 infection in cats and dogs in infected mink farms. Transbound Emerg Dis. 2021.PubMedGoogle Scholar
- van der Leij WJR, Broens EM, Hesselink JW, Schuurman N, Vernooij JCM, Egberink HF. Serological screening for antibodies against SARS-CoV-2 in Dutch shelter cats. Viruses. 2021;13:1634. DOIPubMedGoogle Scholar
- Shou S, Liu M, Yang Y, Kang N, Song Y, Tan D, et al. Animal models for COVID-19: hamsters, mouse, ferret, mink, tree shrew, and non-human primates. Front Microbiol. 2021;12:
626553 . DOIPubMedGoogle Scholar - Hobbs EC, Reid TJ. Animals and SARS-CoV-2: Species susceptibility and viral transmission in experimental and natural conditions, and the potential implications for community transmission. Transbound Emerg Dis. 2021;68:1850–67. DOIPubMedGoogle Scholar
- Patterson EI, Elia G, Grassi A, Giordano A, Desario C, Medardo M, et al. Evidence of exposure to SARS-CoV-2 in cats and dogs from households in Italy. Nat Commun. 2020;11:6231–5. DOIPubMedGoogle Scholar
- Zoccola R, Beltramo C, Magris G, Peletto S, Acutis P, Bozzetta E, et al. First detection of an Italian human-to-cat outbreak of SARS-CoV-2 Alpha variant - lineage B.1.1.7. One Health. 2021;13:
100295 . DOIPubMedGoogle Scholar - Gaudreault NN, Carossino M, Morozov I, Trujillo JD, Meekins DA, Madden DW, et al. Experimental re-infected cats do not transmit SARS-CoV-2. Emerg Microbes Infect. 2021;10:638–50. DOIPubMedGoogle Scholar
- Gaudreault NN, Trujillo JD, Carossino M, Meekins DA, Morozov I, Madden DW, et al. SARS-CoV-2 infection, disease and transmission in domestic cats. Emerg Microbes Infect. 2020;9:2322–32. DOIPubMedGoogle Scholar
- Zhao S, Schuurman N, Li W, Wang C, Smit LAM, Broens EM, et al. Serologic screening of severe acute respiratory syndrome coronavirus 2 infection in cats and dogs during first coronavirus disease wave, the Netherlands. Emerg Infect Dis. 2021;27:1362–70. DOIPubMedGoogle Scholar
- Stevanovic V, Tabain I, Vilibic-Cavlek T, Mauric Maljkovic M, Benvin I, Hruskar Z, et al. The emergence of SARS-CoV-2 within the dog population in Croatia: host factors and clinical outcome. Viruses. 2021;13:1430. DOIPubMedGoogle Scholar
- Hamer SA, Ghai RR, Zecca IB, Auckland LD, Roundy CM, Davila E, et al. SARS-CoV-2 B.1.1.7 variant of concern detected in a pet dog and cat after exposure to a person with COVID-19, USA. Transbound Emerg Dis. 2021;•••:
tbed.14122 ; Epub ahead of print. DOIPubMedGoogle Scholar - Goryoka GW, Cossaboom CM, Gharpure R, Dawson P, Tansey C, Rossow J, et al. One Health investigation of SARS-CoV-2 infection and seropositivity among pets in households with confirmed human COVID-19 cases—Utah and Wisconsin, 2020. Viruses. 2021;13:1813. DOIPubMedGoogle Scholar
- Rotstein DS, Peloquin S, Proia K, Hart E, Lee J, Vyhnal KK, et al. Investigation of SARS-CoV-2 infection and associated lesions in exotic and companion animals. Vet Pathol. 2022;1:
3009858211067467 . DOIPubMedGoogle Scholar - Carpenter A, Ghai RR, Gary J, Ritter JM, Carvallo FR, Diel DG, et al. Determining the role of natural SARS-CoV-2 infection in the death of domestic pets: 10 cases (2020-2021). J Am Vet Med Assoc. 2021;259:1032–9. DOIPubMedGoogle Scholar
- Eckstrand CD, Baldwin TJ, Rood KA, Clayton MJ, Lott JK, Wolking RM, et al. An outbreak of SARS-CoV-2 with high mortality in mink (Neovison vison) on multiple Utah farms. PLoS Pathog. 2021;17:
e1009952 . DOIPubMedGoogle Scholar - Lu L, Sikkema RS, Velkers FC, Nieuwenhuijse DF, Fischer EAJ, Meijer PA, et al. Adaptation, spread and transmission of SARS-CoV-2 in farmed minks and associated humans in the Netherlands. Nat Commun. 2021;12:6802. DOIPubMedGoogle Scholar
- Yen H-L, Sit THC, Brackman CJ, Chuk SSY, Gu H, Tam KWS, et al.; HKU-SPH study team. Transmission of SARS-CoV-2 delta variant (AY.127) from pet hamsters to humans, leading to onward human-to-human transmission: a case study. Lancet. 2022;399:1070–8. DOIPubMedGoogle Scholar
- Hale VL, Dennis PM, McBride DS, Nolting JM, Madden C, Huey D, et al. SARS-CoV-2 infection in free-ranging white-tailed deer. Nature. 2022;602:481–6. DOIPubMedGoogle Scholar
- Palermo PM, Orbegozo J, Watts DM, Morrill JC. SARS-CoV-2 neutralizing antibodies in white-tailed deer from Texas. Vector Borne Zoonotic Dis. 2022;22:62–4.PubMedGoogle Scholar
- Cool K, Gaudreault NN, Morozov I, Trujillo JD, Meekins DA, McDowell C, et al. Infection and transmission of ancestral SARS-CoV-2 and its alpha variant in pregnant white-tailed deer. Emerg Microbes Infect. 2022;11:95–112. DOIPubMedGoogle Scholar
- Barrs VR, Peiris M, Tam KWS, Law PYT, Brackman CJ, To EMW, et al. SARS-CoV-2 in quarantined domestic cats from COVID-19 households or close contacts, Hong Kong, China. Emerg Infect Dis. 2020;26:3071–4. DOIPubMedGoogle Scholar
- Krafft E, Denolly S, Boson B, Angelloz-Pessey S, Levaltier S, Nesi N, et al. Report of one-year prospective surveillance of SARS-CoV-2 in dogs and cats in France with various exposure risks: confirmation of a low prevalence of shedding, detection and complete sequencing of an Alpha variant in a cat. Viruses. 2021;13:1759. DOIPubMedGoogle Scholar
- de Carvalho OV, Ristow LE, Rodrigues DDS, Farias CKDS, Maia RCC. Retrospective surveillance of severe acute respiratory syndrome coronavirus 2 in pets from Brazil. Vet World. 2021;14:2803–8. DOIPubMedGoogle Scholar
- Spada E, Vitale F, Bruno F, Castelli G, Reale S, Perego R, et al. A pre- and during pandemic survey of SARS-CoV-2 infection in stray colony and shelter cats from a high endemic area of northern Italy. Viruses. 2021;13:618. DOIPubMedGoogle Scholar
- Patania OM, Chiba S, Halfmann PJ, Hatta M, Maemura T, Bernard KA, et al. Pulmonary lesions induced by SARS-CoV-2 infection in domestic cats. Vet Pathol. 2021;•••:
3009858211066840 . DOIPubMedGoogle Scholar - Schulz C, Martina B, Mirolo M, Müller E, Klein R, Volk H, et al. SARS-CoV-2–specific antibodies in domestic cats during first COVID-19 wave, Europe. Emerg Infect Dis. 2021;27:3115–8. DOIPubMedGoogle Scholar
- Bartlett SL, Diel DG, Wang L, Zec S, Laverack M, Martins M, et al. SARS-CoV-2 infection and longitudinal fecal screening in Malayan tigers (Panthera tigris Jacksoni), Amur tigers (Panthera tigris Altaica), and African lions (Panthera Leo Krugeri) at the Bronx Zoo, New York, USA. J Zoo Wildl Med. 2021;51:733–44. DOIPubMedGoogle Scholar
- Cossaboom CM, Medley AM, Spengler JR, Kukielka EA, Goryoka GW, Baird T, et al. Low SARS-CoV-2 seroprevalence and no active infections among dogs and cats in animal shelters with laboratory-confirmed COVID-19 human cases among employees. Biology (Basel). 2021;10:898. DOIPubMedGoogle Scholar
- Michelitsch A, Hoffmann D, Wernike K, Beer M. Occurrence of antibodies against SARS-CoV-2 in the domestic cat population of Germany. Vaccines (Basel). 2020;8:772. DOIPubMedGoogle Scholar
- Schulz C, Wylezich C, Wernike K, Gründl M, Dangel A, Baechlein C, et al. Prolonged SARS-CoV-2 RNA shedding from therapy cat after cluster outbreak in retirement home. Emerg Infect Dis. 2021;27:1974–6. DOIPubMedGoogle Scholar
- Semmler G, Traugott MT, Graninger M, Hoepler W, Seitz T, Kelani H, et al. Assessment of S1-, S2-, and NCP-specific IgM, IgA, and IgG antibody kinetics in acute SARS-CoV-2 infection by a microarray and twelve other immunoassays. J Clin Microbiol. 2021;59:e02890–20. DOIPubMedGoogle Scholar
- Pallett SJ, Jones R, Abdulaal A, Pallett MA, Rayment M, Patel A, et al. Variability in detection of SARS-CoV-2-specific antibody responses following mild infection: a prospective multicentre cross-sectional study, London, United Kingdom, 17 April to 17 July 2020. Euro Surveill. 2022;27:
2002076 . DOIPubMedGoogle Scholar - Fritz M, Rosolen B, Krafft E, Becquart P, Elguero E, Vratskikh O, et al. High prevalence of SARS-CoV-2 antibodies in pets from COVID-19+ households. One Health. 2021;11:
100192 . DOIPubMedGoogle Scholar - Perera RAPM, Ko R, Tsang OTY, Hui DSC, Kwan MYM, Brackman CJ, et al. Evaluation of a SARS-CoV-2 surrogate virus neutralization test for detection of antibody in human, canine, cat, and hamster sera. J Clin Microbiol. 2021;59:e02504–20. DOIPubMedGoogle Scholar
- Barroso R, Vieira-Pires A, Antunes A, Fidalgo-Carvalho I. Susceptibility of pets to SARS-CoV-2 infection: lessons from a seroepidemiologic survey of cats and dogs in Portugal. Microorganisms. 2022;10:345. DOIPubMedGoogle Scholar
- Calvet GA, Pereira SA, Ogrzewalska M, Pauvolid-Corrêa A, Resende PC, Tassinari WS, et al. Investigation of SARS-CoV-2 infection in dogs and cats of humans diagnosed with COVID-19 in Rio de Janeiro, Brazil. PLoS One. 2021;16:
e0250853 . DOIPubMedGoogle Scholar - Yaglom HD, Hecht G, Goedderz A, Jasso-Selles D, Ely JL, Ruberto I, et al. Genomic investigation of a household SARS-CoV-2 disease cluster in Arizona involving a cat, dog, and pet owner. One Health. 2021;13:
100333 . DOIPubMedGoogle Scholar - Hosie MJ, Epifano I, Herder V, Orton RJ, Stevenson A, Johnson N, et al.; COVID-19 Genomics UK (COG-UK) consortium. Detection of SARS-CoV-2 in respiratory samples from cats in the UK associated with human-to-cat transmission. Vet Rec. 2021;188:
e247 . DOIPubMedGoogle Scholar - Shi J, Wen Z, Zhong G, Yang H, Wang C, Huang B, et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. 2020;368:1016–20. DOIPubMedGoogle Scholar
- Halfmann PJ, Hatta M, Chiba S, Maemura T, Fan S, Takeda M, et al. Transmission of SARS-CoV-2 in domestic cats. N Engl J Med. 2020;383:592–4. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: May 02, 2022
1Preliminary results from this study were presented at the 30th (September 23–25, 2020) and 31st (July 9–12, 2021) European Congress of Clinical Microbiology and Infectious Diseases.
Table of Contents – Volume 28, Number 6—June 2022
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Dorothee Bienzle, Department of Pathobiology, University of Guelph, Rm 3822, Bldg 89 | 419 Gordon St, Guelph, ON, N1G2W1, Canada
Top