Volume 28, Number 8—August 2022
CME ACTIVITY - Synopsis
Lack of Evidence for Ribavirin Treatment of Lassa Fever in Systematic Review of Published and Unpublished Studies1
Figure 3
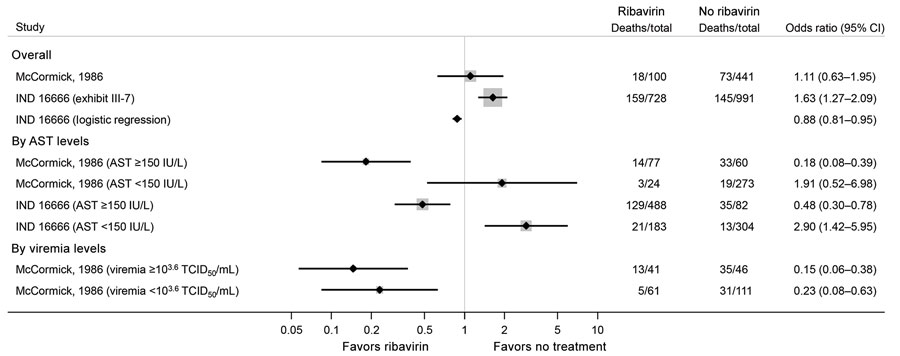
Figure 3. Estimated effects of ribavirin compared with no treatment on mortality outcomes from the McCormick (11) and IND 16666 (Birch & Davis Associates and Sherikon Inc., US Army Medical Research and Development Command, unpub. data, https://media.tghn.org/medialibrary/2019/03/Responsive_Documents_of_Peter_Horby.pdf.pdf; G.V. Ludwig, pers. comm., 2019 March 4, https://media.tghn.org/medialibrary/2019/03/Dr._Ludwig_memo.pdf) studies in a systematic review of published and unpublished studies for evidence for ribavirin treatment of Lassa fever. A horizontal line represents the 95% CI of a study result, with each end of the line representing the boundaries. A point estimate of the study result is represented by a black diamond. A gray box gives a representation of the size of a study compared with all studies in the figure.
References
- Frame JD, Baldwin JM Jr, Gocke DJ, Troup JM. Lassa fever, a new virus disease of man from West Africa. I. Clinical description and pathological findings. Am J Trop Med Hyg. 1970;19:670–6. DOIPubMedGoogle Scholar
- Ogbu O, Ajuluchukwu E, Uneke CJ. Lassa fever in West African sub-region: an overview. J Vector Borne Dis. 2007;44:1–11.PubMedGoogle Scholar
- McCormick JB, Fisher-Hoch SP. Lassa fever. Curr Top Microbiol Immunol. 2002;262:75–109. DOIPubMedGoogle Scholar
- World Health Organization. Lassa fever. 2021 [cited 2022 Mar 22]. https://www.who.int/health-topics/lassa-fever
- McCormick JB, King IJ, Webb PA, Johnson KM, O’Sullivan R, Smith ES, et al. A case-control study of the clinical diagnosis and course of Lassa fever. J Infect Dis. 1987;155:445–55. DOIPubMedGoogle Scholar
- McCormick JB, Webb PA, Krebs JW, Johnson KM, Smith ES. A prospective study of the epidemiology and ecology of Lassa fever. J Infect Dis. 1987;155:437–44. DOIPubMedGoogle Scholar
- Fisher-Hoch SP, Tomori O, Nasidi A, Perez-Oronoz GI, Fakile Y, Hutwagner L, et al. Review of cases of nosocomial Lassa fever in Nigeria: the high price of poor medical practice. BMJ. 1995;311:857–9. DOIPubMedGoogle Scholar
- Fisher-Hoch SP, McCormick JB, Auperin D, Brown BG, Castor M, Perez G, et al. Protection of rhesus monkeys from fatal Lassa fever by vaccination with a recombinant vaccinia virus containing the Lassa virus glycoprotein gene. Proc Natl Acad Sci U S A. 1989;86:317–21. DOIPubMedGoogle Scholar
- Lukashevich IS, Carrion R Jr, Salvato MS, Mansfield K, Brasky K, Zapata J, et al. Safety, immunogenicity, and efficacy of the ML29 reassortant vaccine for Lassa fever in small non-human primates. Vaccine. 2008;26:5246–54. DOIPubMedGoogle Scholar
- CEPI Press Office. CEPI partner INOVIO launches Lassa vaccine phase I trial in West Africa. 2021 Feb 23 [cited 2022 Mar 22]. https://cepi.net/news_cepi/cepi-partner-inovio-launches-lassa-vaccine-phase-i-trial-in-west-africa
- McCormick JB, King IJ, Webb PA, Scribner CL, Craven RB, Johnson KM, et al. Lassa fever. Effective therapy with ribavirin. N Engl J Med. 1986;314:20–6. DOIPubMedGoogle Scholar
- Shaffer JG, Grant DS, Schieffelin JS, Boisen ML, Goba A, Hartnett JN, et al.; Viral Hemorrhagic Fever Consortium. Lassa fever in post-conflict sierra leone. PLoS Negl Trop Dis. 2014;8:
e2748 . DOIPubMedGoogle Scholar - Okokhere P, Colubri A, Azubike C, Iruolagbe C, Osazuwa O, Tabrizi S, et al. Clinical and laboratory predictors of Lassa fever outcome in a dedicated treatment facility in Nigeria: a retrospective, observational cohort study. Lancet Infect Dis. 2018;18:684–95. DOIPubMedGoogle Scholar
- Dahmane A, van Griensven J, Van Herp M, Van den Bergh R, Nzomukunda Y, Prior J, et al. Constraints in the diagnosis and treatment of Lassa Fever and the effect on mortality in hospitalized children and women with obstetric conditions in a rural district hospital in Sierra Leone. Trans R Soc Trop Med Hyg. 2014;108:126–32. DOIPubMedGoogle Scholar
- Shaffer JG, Schieffelin JS, Grant DS, Goba A, Momoh M, Kanneh L, et al.; Viral Hemorrhagic Fever Consortium. Data set on Lassa fever in post-conflict Sierra Leone. Data Brief. 2019;23:
103673 . DOIPubMedGoogle Scholar - Inegbenebor U, Okosun J, Inegbenebor J. Prevention of lassa Fever in Nigeria. Trans R Soc Trop Med Hyg. 2010;104:51–4. DOIPubMedGoogle Scholar
- Ajayi NA, Nwigwe CG, Azuogu BN, Onyire BN, Nwonwu EU, Ogbonnaya LU, et al. Containing a Lassa fever epidemic in a resource-limited setting: outbreak description and lessons learned from Abakaliki, Nigeria (January-March 2012). Int J Infect Dis. 2013;17:e1011–6. DOIPubMedGoogle Scholar
- Salam AP, Cheng V, Edwards T, Olliaro P, Sterne J, Horby P. Time to reconsider the role of ribavirin in Lassa fever. PLoS Negl Trop Dis. 2021;15:
e0009522 . DOIPubMedGoogle Scholar - Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. DOIPubMedGoogle Scholar
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(jul21 1):b2535.
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. DOIPubMedGoogle Scholar
- Hernán MA, Sauer BC, Hernández-Díaz S, Platt R, Shrier I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol. 2016;79:70–5. DOIPubMedGoogle Scholar
- Hamblion EL, Raftery P, Wendland A, Dweh E, Williams GS, George RNC, et al. The challenges of detecting and responding to a Lassa fever outbreak in an Ebola-affected setting. Int J Infect Dis. 2018;66:65–73. DOIPubMedGoogle Scholar
- Allan R, Mardell S, Ladbury R, Pearce E, Skinner K, Saluzzo JF, et al. The progression from endemic to epidemic Lassa fever in war-torn West Africa. In: Saluzzo JF, Dodet B, editors. Emergence and control of rodent-borne viral diseases: hantaviral and arenal diseases: congrès, Annecy, 28–31 Octobre 1998. 1st edition. Paris: Elsevier; 1999. p. 197–205.
- Ehichioya D, Asogun D, Hass M, Becker-Ziaja B, Gunther S, Omilabu S. A retrospective laboratory analysis of clinically diagnosed Lassa fever cases in a tertiary hospital in Nigeria. Int J Infect Dis. 2010;1:e209–10. DOIGoogle Scholar
- Fisher-Hoch SP, Gborie S, Parker L, Huggins J. Unexpected adverse reactions during a clinical trial in rural west Africa. Antiviral Res. 1992;19:139–47. DOIPubMedGoogle Scholar
- Riner A, Chan-Tack KM, Murray JS. Original research: Intravenous ribavirin—review of the FDA’s Emergency Investigational New Drug Database (1997-2008) and literature review. Postgrad Med. 2009;121:139–46. DOIPubMedGoogle Scholar
- Ilesanmi O, Ayodeji O, Abejegah C. Mortality among confirmed Lassa fever cases during the 2017–2019 outbreak in Ondo State, Nigeria [abstract]. Trans R Soc Trop Med Hyg. 2019;113(Suppl 1):S89.
- Asogun DA, Adomeh DI, Ehimuan J, Odia I, Hass M, Gabriel M, et al. Molecular diagnostics for lassa fever at Irrua specialist teaching hospital, Nigeria: lessons learnt from two years of laboratory operation. PLoS Negl Trop Dis. 2012;6:
e1839 . DOIPubMedGoogle Scholar - Bouree P. Les parasitoses intestinales sont encore fréquentes. Med Sante Trop. 2015;25:130. DOIGoogle Scholar
- Buba MI, Dalhat MM, Nguku PM, Waziri N, Mohammad JO, Bomoi IM, et al. Mortality among confirmed Lassa fever cases during the 2015–2016 outbreak in Nigeria. Am J Public Health. 2018;108:262–4. DOIPubMedGoogle Scholar
- Ilori EA, Furuse Y, Ipadeola OB, Dan-Nwafor CC, Abubakar A, Womi-Eteng OE, et al.; Nigeria Lassa Fever National Response Team. Epidemiologic and clinical features of Lassa fever outbreak in Nigeria, January 1–May 6, 2018. Emerg Infect Dis. 2019;25:1066–74. DOIPubMedGoogle Scholar
- Joseph A, Robinson O, Justus E, Matthew N, Chukwuemeka U. Clinical profile of Lassa fever patients in Abakaliki, south-eastern Nigeria, January–March 2018. Ann Med Health Sci Res. 2019;9:598–602.
- Price ME, Fisher-Hoch SP, Craven RB, McCormick JB. A prospective study of maternal and fetal outcome in acute Lassa fever infection during pregnancy. BMJ. 1988;297:584–7. DOIPubMedGoogle Scholar
- Samuels RJ, Moon TD, Starnes JR, Alhasan F, Gbakie M, Goba A, et al. Lassa fever among children in Eastern Province, Sierra Leone: a 7-year retrospective analysis (2012–2018). Am J Trop Med Hyg. 2020;104:585–92. DOIPubMedGoogle Scholar
- Wauquier N, Couffignal C, Manchon P, Smith E, Lungay V, Coomber M, et al. High heart rate at admission as a predictive factor of mortality in hospitalized patients with Lassa fever: An observational cohort study in Sierra Leone. J Infect. 2020;80:671–93. DOIPubMedGoogle Scholar
- Bausch DG, Hadi CM, Khan SH, Lertora JJ. Review of the literature and proposed guidelines for the use of oral ribavirin as postexposure prophylaxis for Lassa fever. Clin Infect Dis. 2010;51:1435–41. DOIPubMedGoogle Scholar
- World Health Organization. Clinical management of patients with viral haemorrhagic fever: a pocket guide for front-line health workers: inerim emergency guidance for country adaptation. 2016 Feb [cited 2022 Mar 22]. https://apps.who.int/iris/bitstream/handle/10665/205570/9789241549608_eng.pdf
- World Health Organization. WHO R&D blueprint. “Efficacy trials of Lassa therapeutics: endpoints, trial design, site selection” WHO workshop—final report. 2018 Apr 25 [cited 2022 Mar 22]. https://www.who.int/blueprint/what/norms-standards/LassaTxeval_FinalmeetingReport.pdf
- Raabe VN, Kann G, Ribner BS, Morales A, Varkey JB, Mehta AK, et al.; Emory Serious Communicable Diseases Unit. Favipiravir and ribavirin treatment of epidemiologically linked cases of Lassa fever. Clin Infect Dis. 2017;65:855–9. DOIPubMedGoogle Scholar
- Eberhardt KA, Mischlinger J, Jordan S, Groger M, Günther S, Ramharter M. Ribavirin for the treatment of Lassa fever: A systematic review and meta-analysis. Int J Infect Dis. 2019;87:15–20. DOIPubMedGoogle Scholar
1Preliminary results from this study were presented at the World Health Organization Emergency Program on December 21, 2019.