Volume 28, Number 8—August 2022
Dispatch
Effectiveness of Naturally Acquired and Vaccine-Induced Immune Responses to SARS-CoV-2 Mu Variant
Figure 2
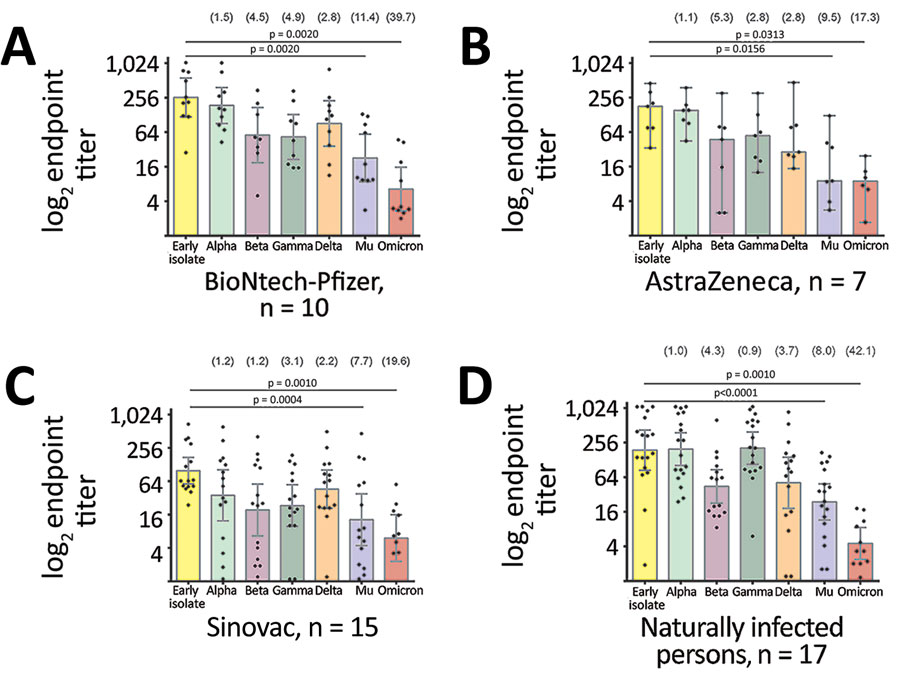
Figure 2. Comparative neutralization of the Mu SARS-CoV-2 variant in Colombia. A–C) Neutralization of SARS-CoV-2 variants from serum samples from persons fully immunized with BNT162b2 (Pfizer-BioNTech, https://www.pfizer.com) (A), AZD1222 (AstraZeneca, https://www.astrazeneca.com) (B), or CoronaVac (Sinovac, http://www.sinovac.com) (C). D) Neutralization of SARS-CoV-2 variants by serum samples from naturally infected persons who tested positive for SARS-CoV-2 antibodies during a seroprevalence study in November 2020. For all panels, each point represents the reciprocal plaque reduction neutralization test endpoint titer of 1 tested serum sample for different SARS-CoV-2 variants; colored bars indicate geometric mean titers, and error bars represent 95% CIs. Values in parentheses above bars represent reduction compared to the parental strain. Statistical significance was determined by the Wilcoxon matched signed-rank test; p values are indicated. For clarity of presentation, only significant values between the early isolate and the Mu variant are shown.
1These first authors contributed equally to this article.