Volume 28, Number 8—August 2022
Dispatch
Effectiveness of Naturally Acquired and Vaccine-Induced Immune Responses to SARS-CoV-2 Mu Variant
Cite This Article
Citation for Media
Abstract
SARS-CoV-2 Mu variant emerged in Colombia in 2021 and spread globally. In 49 serum samples from vaccinees and COVID-19 survivors in Colombia, neutralization was significantly lower (p<0.0001) for Mu than a parental strain and variants of concern. Only the Omicron variant of concern demonstrated higher immune evasion.
Diverse SARS-CoV-2 variants have arisen during the pandemic. As of May 4, 2022, there had been 2 recognized variants of concern (VOC), Delta and Omicron, in addition to earlier emerging VOCs Alpha, Beta, and Gamma and strains previously categorized as variants of interest (VOI). Many VOIs have been understudied in terms of pathogenesis, transmissibility, and potential for immune escape. Delta and Omicron illustrate how variants emerging in tropical settings can spread globally.
Mu was first reported as a VOI in early January 2021 in northern Colombia. While outcompeting other locally circulating variants, Mu spread to additional countries, such as Ecuador, United States, Mexico, and Spain; as of early 2022, it was still circulating at low levels in Colombia (1). Mu caused 70% of all COVID-19 cases in Colombia during May–July 2021 (Figure 1), a period which also accounted for the highest number of deaths in Colombia during the pandemic, suggesting substantial pathogenicity of Mu (1). Mu was later outcompeted by Delta and Omicron, and the number of Mu-related cases gradually decreased through the end of 2021 (Figure 1).
Recent studies relying on data from spike-based pseudovirus testing suggested substantially lower neutralization of Mu compared with the parental B.1 virus in antiserum samples from persons in Japan and China who had received either the BNT162b2 (Pfizer-BioNTech, https://www.pfizer.com) or Sinovac (http://www.sinovac.com) vaccines or recovered from COVID-19 (2,3). Because of inherent limitations in pseudovirus-based systems for reproducing response variations based on natural infection (4), regional differences of immune responses (5), and different vaccines used in Colombia, we comparatively characterized the neutralization of Mu and VOCs using fully infectious viruses and serum samples from persons in Colombia. The study was approved by the Ethics Committee of the Universidad Industrial de Santander (protocol 4110) and by the Ethics Committee of the Charité-Universitätsmedizin Berlin (protocol EA2/031/22). All participants provided written informed consent.
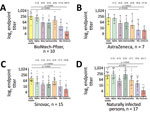
By March 2022, ≈68% of the population of Colombia had been vaccinated, predominantly with spike-based mRNA (BNT162b2), vectored (AZD1222; AstraZeneca, https://www.astrazeneca.com), and chemically inactivated whole virus–based vaccines (CoronaVac) (Appendix Figure 1). To investigate the potency of natural and vaccine-derived immunity, we tested and compared the neutralization activity in 49 serum samples from vaccinated and naturally infected persons in Colombia. Among vaccinated persons, we tested serum from 32 persons sampled in October 2021. Of those, 10 vaccinated with BioNTech-Pfizer were tested a median 99.5 d (range 65–170) after completing vaccination, 7 vaccinated with AstraZeneca were tested a median 146.0 d (range 129–173) after completing vaccination, and 15 vaccinated with CoronaVac were tested a median 46.0 d (range 28–131) after completing vaccination. We tested serum samples from 17 persons who tested positive for SARS-CoV-2 antibodies (MAGLUMI 2019-nCoV IgG; Snibe Diagnostic, https://www.snibe.com) (Table 1; Appendix Table 1) during a seroprevalence study conducted in November 2020. To control whether persons vaccinated with spike-based vaccines were not previously infected, serum samples were tested for IgG against the SARS-CoV-2 nucleocapsid protein by ELISA (SARS-CoV-2 NCP kit; Euroimmun, https://www.euroimmun.com) (Table 2). We used 50% plaque reduction neutralization tests to obtain neutralizing titers against an early isolate and the Alpha, Beta, Delta, Gamma, Omicron BA.1, and Mu variants (Appendix).
Neutralizing antibody titers against Mu were significantly lower than those against the parental isolate (p<0.0001 by Wilcoxon matched-pairs signed-rank test) in all serum samples tested in this study, irrespective of whether immune responses were elicited by vaccination or by natural infection. Vaccine-derived antibodies neutralized Mu on average 8.1-fold (p<0.0001 by Wilcoxon test) less than the parental strain resembling the vaccine backbones (Figure 2, panels A–C; Appendix Figure 2). We found a similar 8.0-fold reduced neutralization of Mu (p<0.0001 by Wilcoxon test) for the group of naturally infected persons (Figure 2, panel D). Despite the relatively lower neutralization potency observed in serum samples from persons immunized with the inactivated full virus-based vaccine Sinovac, observed differences in the ability to neutralize Mu compared with the parental strain among the 3 vaccine groups were not statistically significant (range 7.7–11.4-fold; p = 0.8298 by Kruskal-Wallis test) (Figure 2).
Compared with other variants, neutralizing antibody titers from serum samples of both naturally infected persons and vaccinees were lower against Mu than against all VOCs except for Omicron (Figure 2, panels A and B). Therefore, our results provide strong evidence for immune evasion of the Mu VOI on the basis of results from robust neutralization testing using full viral isolates. Neutralization of Mu by vaccine-induced antibodies was significantly lower than for Beta (p = 0.0083 by Wilcoxon text), for which immune evasion properties led to the suspension of AstraZeneca usage in South Africa (6), and Gamma, which resulted in breakthrough infections in Latin America (7). Immune evasion of Mu is consistent with shared mutations in spike protein residues associated with immune evasion in Beta and Gamma, such as E484K (8). In addition, the mutation leading to the amino acid exchange R346K in Mu is known to be involved in the evasion of monoclonal antibody–mediated neutralization (9), and genomic exchanges occurring at 3 adjacent sites (Y144T, Y145S, and insertion of the amino acid asparagine [N] between spike residues 145 and 146) have been associated with the immune escape properties of Mu (10,11).
Antigenic cartography was recently employed to map the antigenic relationship between the SARS-CoV-2 Omicron and Delta VOCs and other previously circulating VOCs and VOIs (S.H. Wilks et al., unpub. data, https://www.biorxiv.org/content/10.1101/2022.01.28.477987v1). Among the serum samples from Colombia vaccinees, there was a high antigenic distance between Mu and most variants from other serum samples, which clustered together with the parental strain and Alpha (Appendix Figure 3). Of note, antibody responses in naturally infected persons supported past infection with strains bearing similarities to early SARS-CoV-2 isolates and the Gamma variant (Figure 2, panel D). Antibody reactivity in naturally infected persons was thus in concordance with the circulation of SARS-CoV-2 variants in South America during the time of sampling in late 2020 (12), supporting the robustness of our data.
Our study was limited by different time points for sampling of vaccinees and the lack of information on natural infections altering immune responses in vaccinees. However, lack of detectable N-protein antibody responses and the absence of clinical records suggestive of COVID-19 infection in vaccinees immunized with spike-based vaccines supports the robustness of our data despite the vaccinees’ unclear infection histories.
Our data highlight the importance of continuous monitoring for the emergence of new SARS-CoV-2 variants and strains and the timely identification of those variants with potential to evade naturally elicited and vaccine-derived immune responses, using local sampling specimens in the context of regional epidemiologic conditions. Moreover, our data confirmed the potential of Mu to partially evade immune responses, which may affect the efficacy of vaccination programs in southern America and other areas (7,13). Further studies are warranted to evaluate the pathogenicity of and cell-mediated immunity against Mu and the ability of immune responses associated with Mu to neutralize other SARS-CoV-2 variants. However, because vaccination boosters still provide some degree of protection against severe disease from Omicron (3,14), which shows more immunity evasion than Mu, vaccination will likely still provide protection against severe disease from Mu.
Dr. Oliveira-Filho is a virologist at the Institute of Virology, Charité Universitätsmedizin Berlin. His research interests include the epidemiology and evolution of emerging viruses.
Acknowledgments
We thank Victor Carvalho Urbieta, Ana María Arboleda, Karina Freyle, and Arne Kühne for their technical support. The Gamma and the Omicron SARS-CoV-2 isolates were obtained from the European Virus Archive) and provided by Dr. Chantal Reusken from the National Institute for Public Health and the Environment.
This work was supported by the Deutsche Gesellschaft für Internationale Zusammenarbeit GmbH (project nos. 88114108 and 81263203), Universidad Industrial de Santander, and MinCiencias-SGR (project no. BPIN 2020000100126).
References
- Laiton-Donato K, Franco-Muñoz C, Álvarez-Díaz DA, Ruiz-Moreno HA, Usme-Ciro JA, Prada DA, et al. Characterization of the emerging B.1.621 variant of interest of SARS-CoV-2. Infect Genet Evol. 2021;95:
105038 . DOIPubMedGoogle Scholar - Uriu K, Kimura I, Shirakawa K, Takaori-Kondo A, Nakada TA, Kaneda A, et al.; Genotype to Phenotype Japan (G2P-Japan) Consortium. Neutralization of the SARS-CoV-2 Mu variant by convalescent and vaccine serum. N Engl J Med. 2021;385:2397–9. DOIPubMedGoogle Scholar
- Wang Y, Ma Y, Xu Y, Liu J, Li X, Chen Y, et al. Resistance of SARS-CoV-2 Omicron variant to convalescent and CoronaVac vaccine plasma. Emerg Microbes Infect. 2022;11:424–7. DOIPubMedGoogle Scholar
- Chen M, Zhang XE. Construction and applications of SARS-CoV-2 pseudoviruses: a mini review. Int J Biol Sci. 2021;17:1574–80. DOIPubMedGoogle Scholar
- Kollmann TR. Variation between populations in the innate immune response to vaccine adjuvants. Front Immunol. 2013;4:81. DOIPubMedGoogle Scholar
- Madhi SA, Izu A, Pollard AJ. ChAdOx1 nCoV-19 vaccine efficacy against the B.1.351 variant. [Reply]. N Engl J Med. 2021;385:571–2. DOIPubMedGoogle Scholar
- Vignier N, Bérot V, Bonnave N, Peugny S, Ballet M, Jacoud E, et al. Breakthrough infections of SARS-CoV-2 Gamma variant in fully vaccinated gold miners, French Guiana, 2021. Emerg Infect Dis. 2021;27:2673–6. DOIPubMedGoogle Scholar
- Cai Y, Zhang J, Xiao T, Lavine CL, Rawson S, Peng H, et al. Structural basis for enhanced infectivity and immune evasion of SARS-CoV-2 variants. Science. 2021;373:642–8. DOIPubMedGoogle Scholar
- McCallum M, Czudnochowski N, Rosen LE, Zepeda SK, Bowen JE, Walls AC, et al. Structural basis of SARS-CoV-2 Omicron immune evasion and receptor engagement. Science. 2022;375:864–8. DOIPubMedGoogle Scholar
- Hossain MJ, Rabaan AA, Mutair AA, Alhumaid S, Emran TB, Saikumar G, et al. Strategies to tackle SARS-CoV-2 Mu, a newly classified variant of interest likely to resist currently available COVID-19 vaccines. Hum Vaccin Immunother. 2022;18:
2027197 . DOIPubMedGoogle Scholar - Uriu K, Cardenas P, Munoz E, Barragan V, Kosugi Y, Shirakawa K, et al. Characterization of the immune resistance of SARS-CoV-2 Mu variant and the robust immunity induced by Mu infection. J Infect Dis. 2022:jiac053.
- Gutierrez B, Marquez S, Prado-Vivar B, Becerra-Wong M, Guadalupe JJ, Candido DDS, et al. Genomic epidemiology of SARS-CoV-2 transmission lineages in Ecuador. Virus Evol. 2021;7:veab051.
- Collie S, Champion J, Moultrie H, Bekker LG, Gray G. Effectiveness of BNT162b2 vaccine against Omicron variant in South Africa. N Engl J Med. 2022;386:494–6. DOIPubMedGoogle Scholar
- Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–11. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: July 13, 2022
1These first authors contributed equally to this article.
Table of Contents – Volume 28, Number 8—August 2022
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Jan Felix Drexler, Institute of Virology, Charitéplatz 1, 10117 Berlin, Germany
Top